The aldol addition is an important, simple reaction for the formation of C–C bonds. Small substrates, however, can be challenging for this reaction because the product is very similar to the reactant and has the same aldehyde functional group. This can cause the desired product to react again under the same conditions and lead to unwanted oligo- or polymerizations.
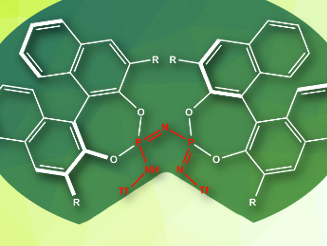
Benjamin List and colleagues, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, have designed a strongly acidic chiral imidodiphosphorimidate catalyst (IDPi, pictured), which can circumvent this problem. The active, acidic site of the catalyst (pictured in red) is confined by the sterically demanding substituents, like the active site in many enzymes. This allows the catalyst to differentiate between the smaller reactant and the slightly larger product and improves the selectivity for single aldolizations.
The team used the catalyst for the Mukaiyama aldol reaction between the triethylsilyl (TES) or tert-butyldimethylsilyl (TBS) enolates of acetaldehyde and a range of acceptor aldehydes. Due to the chirality of the catalyst, the reaction can be performed in an enantioselective manner. The desired aldol products were obtained in high yields and enantioselectivities.
- Confined acids catalyze asymmetric single aldolizations of acetaldehyde enolates,
Lucas Schreyer, Philip S. J. Kaib, Vijay N. Wakchaure, Carla Obradors, Roberta Properzi, Sunggi Lee, Benjamin List,
Science 2018, 362, 216–219.
https://doi.org/10.1126/science.aau0817