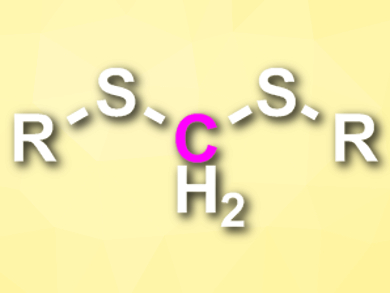
The greenhouse gas carbon dioxide can serve as a useful chemical feedstock. Most methods for the incorporation of CO2 into valuable chemicals are based on a two- or six-electron reduction of CO2. The four-electron reduction of CO2, in contrast, is rarely used in organic synthesis.
Chanjuan Xi, Tsinghua University, Beijing, China, and colleagues have developed a selective four-electron reduction of CO2 in the presence of thiols to generate dithioacetals (pictured). The team reacted a range of aromatic and aliphatic thiols with CO2 (1 atm), using NaBH4 as a reductant and 1,4-dioxane as the solvent. Iodine was used as an additive.
The desired thioacetals were obtained in moderate to excellent yields and the reaction could be scaled up to the gram scale. The developed reaction selectively converts CO2 into a methylene unit and forms two C–S bonds simultaneously. According to the researchers, future work will focus on the reaction mechanism.
- Reduction of CO2 into Methylene Coupled with the Formation of C–S Bonds under NaBH4/I2 System,
Zhiqiang Guo, Bo Zhang, Xuehong Wei, Chanjuan Xi,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b02730