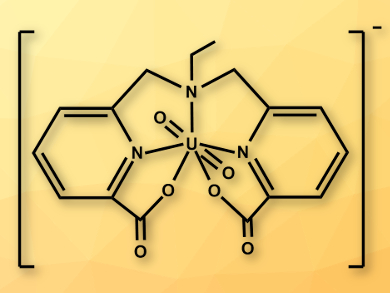
Uranyl(V) (UO2+) is unstable in water and disproportionates into U(VI) and U(IV) species. Due to this instability, the role of uranyl(V) in uranium chemistry has been difficult to investigate. Studies of uranyl(V) species have only been possible in acidic solutions, where the disproportionation is slower, or in the narrow pH range in which its carbonate complex is relatively stable (pH = 11.7–12). Ligands which can stabilize uranyl(V) in neutral aqueous solutions had not been found so far.
Marinella Mazzanti and colleagues, Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland, have synthesized a uranyl(V) complex with a polydentate ligand (pictured) that is stable in water at pH 7–10. The team reacted [UO2(NO3)2(H2O)6] with H2dpaea (H2dpaea = bis(pyridyl-6-methyl-2-carboxylate)-ethylamine) in methanol to prepare the uranyl(IV) precursor complex [UO2(dpaea)]. The desired uranyl(V) complex [UO2(dpaea)]– was then prepared by reduction in pyridine.
The resulting complex can be dissolved in water and is stable with respect to ligand dissociation and disproportionation for days to weeks, depending on the pH. This was shown by 1H NMR spectroscopy in D2O. At pH values below 7, the complex is protonated and disproportionates. According to the researchers, the synthesized system could be a valuable tool for investigating the reduction of uranyl species under environmental conditions.
- Synthesis and Characterization of a Water Stable Uranyl(V) Complex,
Radmila Faizova, Rosario Scopelliti, Anne-Sophie Chauvin, Marinella Mazzanti,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b07885