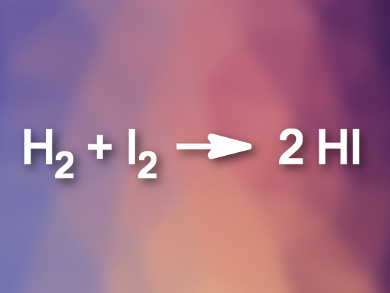Iodoalkanes are important synthetic intermediates in organic chemistry. They can be prepared by the addition of hydrogen iodide (HI) to alkenes. However, aqueous HI is not very reactive and gaseous HI can be unstable. Thus, HI is ideally generated in situ.
Jingchao Chen, Baomin Fan, and colleagues, Yunnan Minzu University, Kunming, China, have developed a method for the direct preparation of anhydrous HI from H2 and I2 using a rhodium catalyst. The resulting HI can be used for the synthesis of iodoalkanes. The team used Rh(COD)BF4 (COD = 1,5-cyclooctadiene), together with phosphine ligands, as a catalyst to generate HI from I2 and gaseous H2. The HI reacted directly with a range of alkenes in toluene to give the desired iodoalkanes.
The method can also be used for reactions of other substrates with HI, e.g., aldehydes, alcohols, and cyclic ethers. The team proposes a mechanism involving a hydrogen molecule dissociation, followed by iodine acting as an atomic hydrogen acceptor, resulting in the generation of anhydrous HI. According to the researchers, the developed approach is also promising for further reactions involving HI.
- Rhodium-Catalyzed Generation of Anhydrous Hydrogen Iodide: An Effective Method for the Preparation of Iodoalkanes,
Chaoyuan Zeng, Guoli Shen, Fan Yang, Jingchao Chen, Xuexin Zhang, Cuiping Gu, Yongyun Zhou, Baomin Fan,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b02980