Metal–organic frameworks (MOFs) are composed of metal nodes and organic linkers. Usually, the positive charge of a metal cation is neutralized by the immediately surrounding anionic ligands. Ionic MOFs, in contrast, contain either positive or negative isolated charges, which can cause improved interactions between the MOF and polar or polarizable species.
However, in ionic MOFs, the free counterions (which are needed to balance the isolated charges) occupy pores in the MOF and reduce its performance. Zwitterionic or charge-separated MOFs can solve this problem by combining both positive and negative isolated charges in the MOF structure itself.
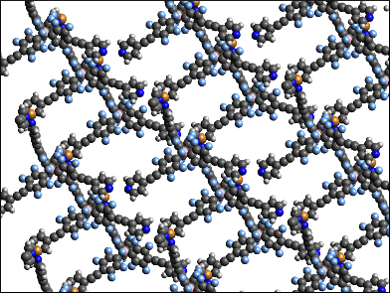
Gayan Rubasinghege, New Mexico Institute of Mining and Technology, Socorro, USA, Yang Qin, University of New Mexico, Albuquerque, USA, and colleagues have synthesized a new charge-separated MOF called UNM-1 (pictured). The team combined a large tetrahedral anionic borate ligand with Cu+ ions to assemble the MOF. The borate ligand has four tetrafluorophenylethynyl pyridine groups, which serve to separate the negative charge at the boron atoms and the positive charge at the copper ions. The tetrahedral structure of the borate’s binding sites leads to a diamond-like structure for the MOF.
Since there are no free counterions in the pores, the synthesized MOF has a relatively large free surface area. The material is stable under ambient conditions. It has properties that could be useful for the separation of CO2 from other gases, e.g., an ideal CO2/N2 selectivity of 99 at 313 K and 1 bar. According to the researchers, modifying the length and structure of the four substituents at the borate could lead to a range of charge-separated MOFs with tailored properties.
- A charge-separated diamondoid metal–organic framework,
Sheela Thapa, Eshani Hettiarachchi, Diane A. Dickie, Gayan Rubasinghege, Yang Qin,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc07098a