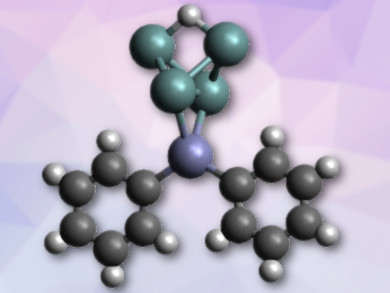
Protonated Zintl-type clusters of group 14 elements (tetrels, E) are rare. This is in spite of the fact that these clusters, e.g., E44–, are highly basic. Examples of E44– ions as ligands in transition-metal complexes are also rare.
Thomas F. Fässler and colleagues, Technical University of Munich, Garching, German, have synthesized a diphenylzinc complex of the protonated tetrahedral tetrel cluster anion [Ge4]4–, i.e., [HGe4ZnPh2]3– (pictured). The team used crown ethers to capture the counterions and, thus, improve solubility. They combined K6Rb6Ge17, ZnPh2, and [18]crown-6 in liquid ammonia and obtained [K([18]crown-6)][Rb([18]crown-6)]2[HGe4ZnPh2]·8 NH3. The four germanium ions (pictured in green) form a distorted tetrahedron, which is protonated on one side and bound to zinc (pictured in violet) on the other.
A single-crystal structure determination of the compound showed equal Ge–H bond lengths and a widening of the bridged Ge–Ge bond. This structural data and the results of density functional theory (DFT) calculations provide evidence for the first three-center two-electron Ge–H–Ge bond. The compound also represents the first example of a protonated [Ge4]4– cluster.
- [(μ2-H)(η2-Ge4)ZnPh2]3–, an edge-on protonated E4 cluster establishing the first three-center two-electron Ge–H–Ge bond,
Thomas Henneberger, Wilhelm Klein, Jasmin V. Dums, Thomas F. Fässler,
Chem. Commun. 2018, 54, 12381–12384.
https://doi.org/10.1039/c8cc06843g