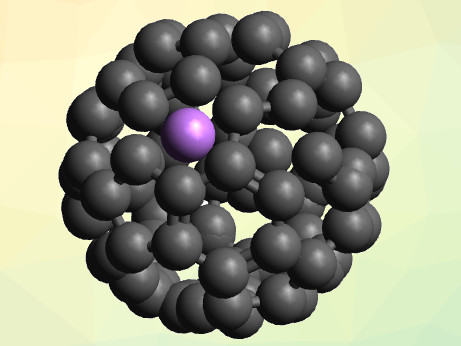
In endohedral fullerenes, metal atoms are captured inside the fullerene carbon cages. The most common endohedral fullerenes are created by growing the carbon cage around metal atoms. In many cases, the most energetically favorable endohedral fullerene cage is structurally different from its equivalent empty cage. More information is needed to understand why endrohedral fullerenes act differently.
Eleanor E. B. Campbell, University of Edinburgh, UK, and Konkuk University, Seoul, South Korea, and colleagues have used a combination of gas phase Rydberg photoelectron spectroscopy, scanning tunneling spectroscopy (STS), and time-dependent density functional theory (TD-DFT) to study Li@C60 endohedral fullerenes (pictured).
The researchers investigated the diffuse, low-lying, Rydberg-like “Super-Atom Molecular Orbitals” (SAMOs) in Li@C60. These orbitals have also been studied for pure C60, so a direct comparison could be made to study the effect that the lithium ions have on the formation of the fullerene.
It was found that the presence of the lithium ion within the fullerene cage causes a big distortion in some energetically low-lying SAMOs, depending on the position of the lithium ion inside the cage. At high temperatures, the lithium ion is mobile and changes position, but at low temperatures, the lithium ion is locked in place in an asymmetric, off-center position. In this case, the energy levels of the low-lying SAMOs are markedly different from those in empty C60 cages. According to the researchers, the dependence of the energy levels on the lithium’s position in the cage could be used to build a molecular switch.
- Angle-resolved photoelectron spectroscopy and scanning tunnelling spectroscopy studies of the endohedral fullerene Li@C60,
M. Stefanou, H. J. Chandler, B. Mignolet, E. Williams, S. A. Nanoh, J. O. F. Thompson, F. Remacle, R. Schaub, E. E. B. Campbell,
Nanoscale 2018.
https://doi.org/10.1039/c8nr07088a