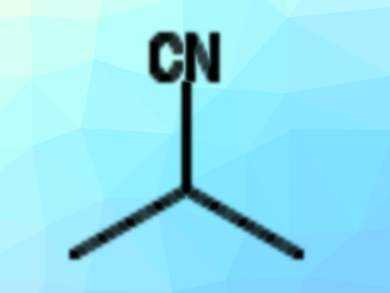
Nitriles (pictured) are useful precursors for many types of compounds in organic synthesis. They can be prepared by nucleophilic substitution reactions of, e.g., alkyl halides with cyanide. However, the cyanide sources used for this are often very toxic. In addition, such substitution reactions at unactivated secondary alkyl halides can be challenging.
Yuanhong Liu and colleagues, Shanghai Institute of Organic Chemistry, University of the Chinese Academy of Sciences, have developed a nickel-catalyzed cyanation reaction of unactivated secondary alkyl chlorides or bromides which uses Zn(CN)2 as the cyanide source. Compared with standard reagents such as NaCN or KCN, Zn(CN)2 has a lower toxicity. The team used a variety of alkyl chlorides and bromides as substrates, NiCl2·6H2O as the catalyst, Xantphos (4,5-bis(diphenylphosphino)-9,9-dimethylxanthene) as a ligand, DMAP (4-dimethylaminopyridine) and n-Bu4NCl as additives, and CH3CN as the solvent.
The reaction gives the desired alkyl nitriles in good to high yields. A range of functional groups is tolerated. The team proposes a radical reaction mechanism. According to the researchers, the developed reaction is the first thermally driven metal-catalyzed cyanation of unactivated alkyl halides.
- Nickel-Catalyzed Cyanation of Unactivated Alkyl Chlorides or Bromides with Zn(CN)2,
Aiyou Xia, Xin Xie, Haoyi Chen, Jidong Zhao, Chunli Zhang, Yuanhong Liu,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03539