Pyrene is a very well-known chromophore. Its derivatives have applications in, e.g., dyes or optoelectronics. Pyrene is easy to functionalize in the 1,3,6,8-positions. However, the opposite substitution pattern, i.e., the synthesis of 2,4,5,7,9,10-substituted pyrenes, is harder to achieve.
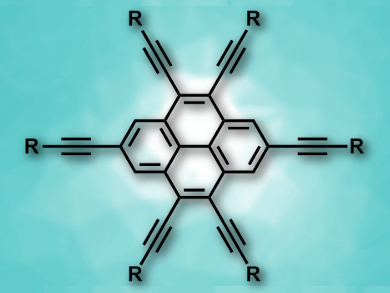
Ethynyl substituents are particularly useful in pyrene chemistry because they extend the π-conjugated system and allow tuning of the optical properties. 4,5,9,10-Tetraethynylpyrenes and 2,4,5,9,10-pentaethynylpyrenes are known, but there had been no synthesis of 2,4,5,7,9,10-hexaethynylpyrenes (pictured) so far.
Shin-ichiro Kato, University of Shiga Prefecture, Japan, Yosuke Nakamura, Gunma University, Japan, and colleagues have achieved the first synthesis of 2,4,5,7,9,10-hexaethynylpyrenes (pictured, R = Si(iPr)3, C6H5–tBu, C6H5-ODec, or tBu). The team started from pyrene-4,5-dione, which was brominated to give 2,7,9,10-tetrabromopyrene-4,5-dione. This was followed by a nucleophilic addition of lithium acetylides of different alkynes to the two carbonyl groups. A Migita–Kosugi–Stille coupling was used to introduce the remaining four ethynyl substituents. In the final step, a reductive aromatization gave the desired hexaethynylpyrenes.
The synthesized 2,4,5,7,9,10-hexaethynylpyrenes have distinctly different optical and electronic properties from the corresponding tetraethynylpyrenes and diethynylpyrenes. The compounds with C6H5-R’-type substituents can self-assemble in solution, which is caused by π–π stacking interactions. According to the researchers, other functionalizations of the hexaethynylpyrene framework could allow the tuning of the compounds’ properties and the development of new π-functional materials.
- 2,4,5,7,9,10-Hexaethynylpyrenes: Synthesis, Properties, and Self-Assembly,
Shin-ichiro Kato, Haruka Kano, Ken-ichi Irisawa, Naoki Yoshikawa, Ryuichiro Yamamoto, Chitoshi Kitamura, Daiki Nara, Takeshi Yamanobe, Hiroki Uehara, Yosuke Nakamura,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03290



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)