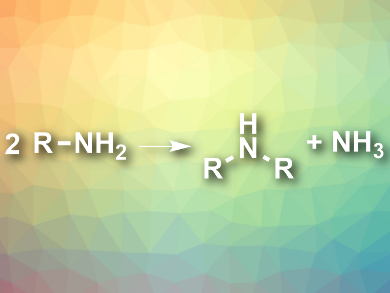Symmetric secondary amines (R2NH) occur in, e.g, agrochemicals, drugs, or catalysts. They can be synthesized from the corresponding primary amines in an atom-economical way by self-condensation (reaction pictured). The only byproduct in this reaction is ammonia, and organic waste is avoided. However, ambient-temperature variants of this reaction had not been developed so far.
Susumu Saito, Hiroshi Naka, Nagoya University, Japan, and colleagues have achieved the first photocatalytic self-condensation of primary amines at ambient temperature. The team prepared a Pd/TiO2 photocatalyst from PdCl2(CH3CN)2, TiO2, and NaBH4. This catalyst was then used to convert primary amines to the corresponding secondary amines under irradiation with UV light in cyclopentyl methyl ether (CPME) as a solvent.
The reaction gives the desired products at moderate to excellent isolated yields at a temperature of 30 °C. The proposed reaction mechanism involves an oxidation of the primary amine to an imine by holes on the photocatalyst surface, an amine–imine exchange between this imine and another primary amine, and a reduction of the formed secondary imine to the secondary amine. According to the researchers, the approach complements existing methods for secondary amine synthesis.
- Pd/TiO2-Photocatalyzed Self-Condensation of Primary Amines To Afford Secondary Amines at Ambient Temperature,
Lyu-Ming Wang, Kensuke Kobayashi, Mitsuhiro Arisawa, Susumu Saito, Hiroshi Naka,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03271