Expanded porphyrin-based compounds can have useful properties that simple porphyrin rings cannot support, for example, structural flexibility or aromaticity that can be switched on or off by external stimuli. Carbaporphyrins are a type of porphyrin analogue in which one or more nitrogen atoms in the central ring are replaced by carbon atoms.
Dongho Kim, Yonsei University, Seoul, South Korea, Jonathan L. Sessler, The University of Texas at Austin, USA, and colleagues have synthesized the first 3D carbaporphyrin cage. The compound was prepared via a [2+4] condensation reaction between a dibenzo[g,p]chrysene tetrapyrrole derivative and pentafluorobenzaldehyde, followed by an oxidation using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ).
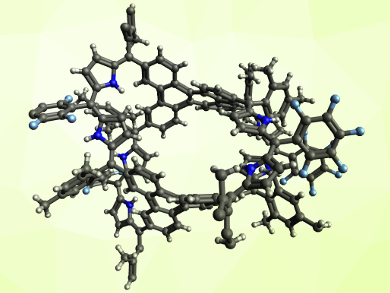
The fully conjugated, cage-like product is non-aromatic, but can be converted into an aromatic system (pictured) by protonation with trifluoroacetic acid (TFA). The aromatic character has been confirmed by NMR spectroscopy. In the team’s approach, the porphyrinoid ring is created during the formation of the expanded system from an open precursor. This method complements earlier synthesis strategies that connect existing porphyrinoids.
- Three-Dimensional Fully Conjugated Carbaporphyrin Cage,
Xian-Sheng Ke, Taeyeon Kim, Qing He, Vincent M. Lynch, Dongho Kim, Jonathan L. Sessler,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b11158



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)