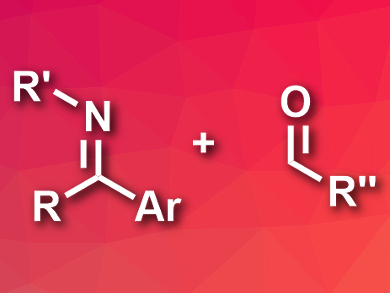
Imines (pictured left) usually act as electrophiles in organic reactions. Their reactivity can, however, be inverted, e.g., by activation with metals or deprotonation. Photoredox catalysis can also be used to activate imines, but this rarely results in nucleophilic reactivity.
Patrick J. Walsh, Nanjing Tech University, China, and University of Pennsylvania, Philadelphia, USA, and colleagues have developed a cross-coupling reaction between imines and aldehydes under photoredox catalysis in which the imines act as nucleophiles. The team used a variety of ketimines and aldehydes as reactants, N,N-dicyclohexylmethylamine (Cy2NMe) as an electron donor, [Ir(ppy)2(4,4′-tBu-bpy)]PF6 (ppy = 2-phenylpyridine, bpy = 2,2′-bipyridine) as a catalyst, and acetonitrile as a solvent.
The desired coupling products, amino alcohols, were obtained in good to excellent yields under blue-light irradiation. The researchers propose a reaction mechanism in which Cy2NMe is oxidized by the iridium catalyst to form a radical cation. This radical activates the ketimine, which is reduced by the catalyst to give a carbanion. This carbanion can, in turn, attack the aldehyde in a nucleophilic addition and form the desired new C–C bond.
- Reductive Cross-Coupling of Aldehydes and Imines Mediated by Visible Light Photoredox Catalysis,
Rui Wang, Mengyue Ma, Xu Gong, Xinyuan Fan, Patrick J. Walsh,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03394