Barium is abundant in the earth’s crust. This poses a problem for the petroleum industry: Barium-containing water occurs naturally in the ground and can form insoluble barium sulfate scale in pipes. This scale is usually removed using chelating ligands that capture barium ions. However, the commonly used ligands have only low affinities for Ba2+. Harsh conditions such as high pH and heat are, thus, required for scale removal.
Justin J. Wilson, Cornell University, Ithaca, NY, USA, and colleagues have found a macrocyclic ligand with a high affinity for Ba2+ for the removal of barium sulfate scale under mild conditions. The team investigated the properties of three diaza-18-crown-6 macrocycles with different substituents (picolinate, 8-hydroxyquinoline, or a sulfonate-modified 8-hydroxyquinoline). The stability constants of the barium complexes were measured by potentiometric titration.
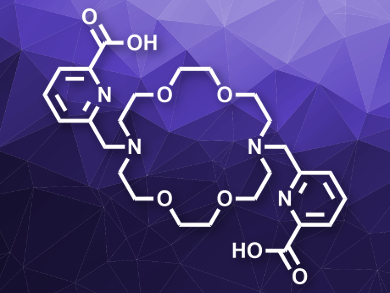
The ligand with two picolinate arms (“macropa”, pictured) has the highest affinity for Ba2+ at pH 7.4 reported thus far. The team tested the ligand’s suitability for dissolving barium sulfate scale and found complete dissolution of BaSO4 in a suspension after 30 min at pH 8 and room temperature. The ligand can be recovered in acidic solution and reused. According to the researchers, macropa is promising for the removal of barium sulfate scale deposits in the petroleum industry.
- Rapid Dissolution of BaSO4 by Macropa, an 18-Membered Macrocycle with High Affinity for Ba2+,
Nikki A. Thiele, Samantha N. MacMillan, Justin J. Wilson,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b08704