α-Substituted benzylamines can be found in a variety of biologically active compounds. Usually, functionalized benzyl derivatives are required for the formation of the C–N bond during the synthesis of this type of molecule. The existing direct routes to primary amines usually afford only protected amines and require further steps to obtain the desired product.
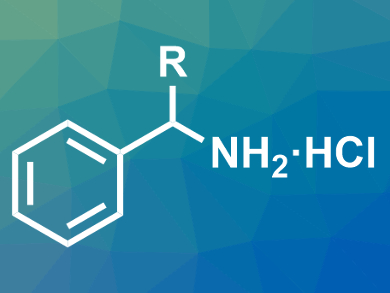
Søren Kramer, Technical University of Denmark, Lyngby, has developed a direct synthesis of α-substituted primary benzylamines from alkylarenes and diarylimines. The reaction directly gives the desired amines as their hydrochloride salts (pictured). The alkylarenes were combined with benzophenone imine using CuI as a catalyst, 1,10-phenanthroline as a ligand, (tBuO)2 as a stable peroxide, and chlorobenzene as the solvent.
The desired unprotected amines were obtained in good yields. The catalyst system is simple and can be used in low concentrations. The reaction tolerates both air and moisture. Overall, the protocol is a practical, fast path to primary benzylamine building blocks.
- Synthesis of α-Substituted Primary Benzylamines through Copper-Catalyzed Cross-Dehydrogenative Coupling,
Søren Kramer,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b03505



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)