Cyclic compounds of elements such as silicon have interesting reactivities and bonding modes. Silicon-based analogues of, e.g., cyclohexanes or benzenes are known. Similarly, there are compounds containing rings of aluminium atoms. However, no molecular cyclic compounds containing both silicon and aluminium had been reported so far.
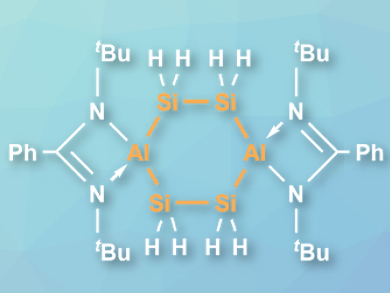
Hongping Zhu, Xiamen University, China, Dietmar Stalke, Herbert W. Roesky, University of Göttingen, Germany, Debasis Koley, Indian Institute of Science Education and Research (IISER), Kolkata, and colleagues have synthesized the first heterocycle of this type, (PhC(NtBu)2Al)2(SiH2)4 (pictured). The team reacted the disilylene (PhC(NtBu)2)Si–Si(PhC(NtBu)2) with AlH3·NEtMe2 in a ratio of 2:3 in toluene at –78 °C to room temperature.
The product was obtained as a crystalline solid in a yield of 18 %. It is stable in the solid state in the absence of air and moisture. The compound was characterized using 29Si NMR spectroscopy and single-crystal X-ray diffraction. The team found that the Si4Al2 ring has a centrosymmetric structure with a boat conformation, analogous to cyclohexane. According to the researchers, the reaction proceeds via the insertion of the low-valent silicon atoms in the disilylene into Al–H bonds, followed by rearrangement of the ligands from Si to Al, and the migration of hydrides from Al to Si.
- (PhC(NtBu)2Al)2(SiH2)4 Six-Membered Heterocycle: Comparable in Structure to Cyclohexane,
Herbert W. Roesky, Jiancheng Li, Zhong Mingdong, Helena Keil, Hongping Zhu, Regine Herbst-Irmer, Dietmar Stalke, Debasis Koley, Sriman De,
Chem. Commun. 2019.
https://doi.org/10.1039/c8cc10124h