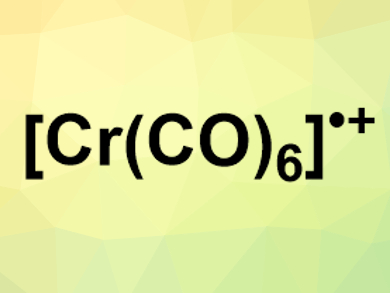Transition-metal carbonyl complexes are well-studied compounds. Neutral and anionic complexes with only CO ligands are very common, but their cationic analogues are more challenging to make, and open-shell variants of transition-metal carbonyl cations had never been synthesized before.
Frank Breher, Karlsruhe Institute of Technology (KIT),Germany, Ingo Krossing, University of Freiburg, Germany, and colleagues have synthesized the first two stable open-shell [Cr(CO)6]•+ salts. The team oxidized Cr(CO)6 with either [NO]+[Al(OR)4]− or [NO]+[F-{Al(OR)3}2]− (R = C(CF3)3)) in dichloromethane and removed the resulting NO gas using a vacuum. The anions are weakly coordinating to avoid reactions with the cations. The desired salts, [Cr(CO)6]•+[Al(OR)4]− and [Cr(CO)6]•+[F-{Al(OR)3}2]−, were obtained in near quantitative yields in solid form.
The salts are stable at room temperature under inert conditions for months. The researchers chracterized the compounds using infrared (IR), Raman, electron paramagnetic resonance (EPR), and NMR spectroscopy as well as X-ray diffraction. They found that the structure of the cation is closely related to the well-known isoelectronic and isostructural V(CO)6.
- Stable salts of the hexacarbonyl chromium(I) cation and its pentacarbonyl-nitrosyl chromium(I) analogue,
Jan Bohnenberger, Wolfram Feuerstein, Daniel Himmel, Michael Daub, Frank Breher, Ingo Krossing,
Nat. Commun. 2019.
https://doi.org/10.1038/s41467-019-08517-2