Photoelectrochemical (PEC) cells split water into hydrogen and oxygen using a photocathode and a photoanode. PEC cells can work under mild conditions with light. In principle, this also makes them suitable for catalyzing other reactions. However, PEC cells have rarely been used in organic synthesis so far. Direct amination is useful for the synthesis of pharmaceuticals and agrochemicals. It usually requires high temperatures and needs a directing group.
Xile Hu, École Polytechnique Fédérale de Lausanne (EPFL), Switzerland, and colleagues have developed a simple method for aminating arenes without the need for a directing group. Based on a PEC cell, the method catalyzes the reaction using the low-cost, earth-abundant semiconductor hematite as a photoanode. Hematite was found to work well for direct amination under visible light, while its high stability promises a long lifetime as a working catalyst.
The developed photoelectrocatalysis process consumes less energy than direct electrocatalysis. Under illumination, the photogenerated holes in haematite oxidize electron-rich arenes to radical cations. These react further with azoles to give useful nitrogen heterocycles. An unusual ortho selectivity was achieved, probably due to a hydrogen-bonding interaction between the substrates and the hexafluoroisopropanol co-solvent.
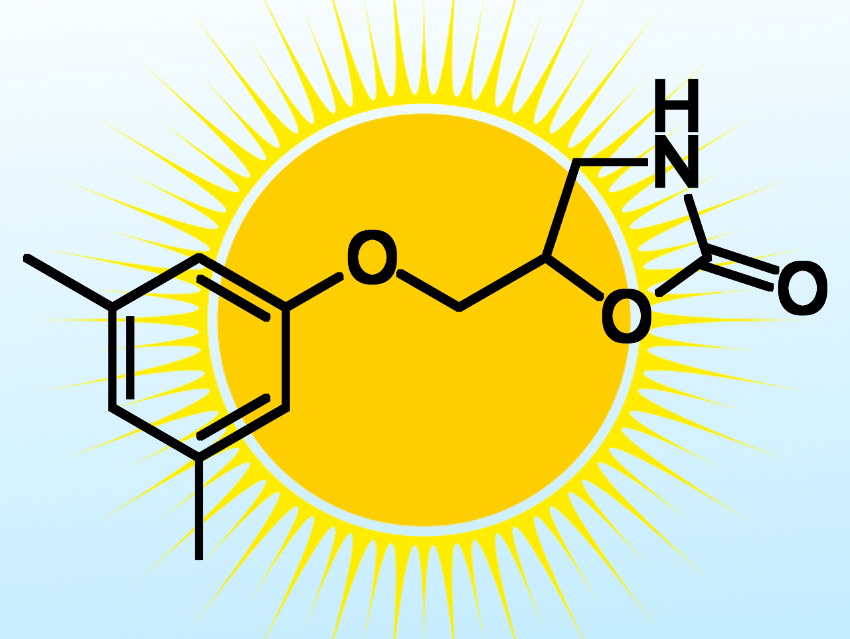
The researchers used their method to make several pharmaceutically active molecules, including derivatives of the muscle relaxant metaxalone (pictured) and the antimicrobial benzethonium chloride. According to the researchers, their work merges two traditionally separated fields, namely photoelectrochemistry and organic synthesis, and demostrates the potential of using PEC cells for the production of value-added chemicals and pharmaceuticals.
- Photoelectrocatalytic arene C–H amination,
Lei Zhang, Laurent Liardet, Jingshan Luo, Dan Ren, Michael Grätzel, Xile Hu,
Nat. Catal. 2019.
https://doi.org/10.1038/s41929-019-0231-9