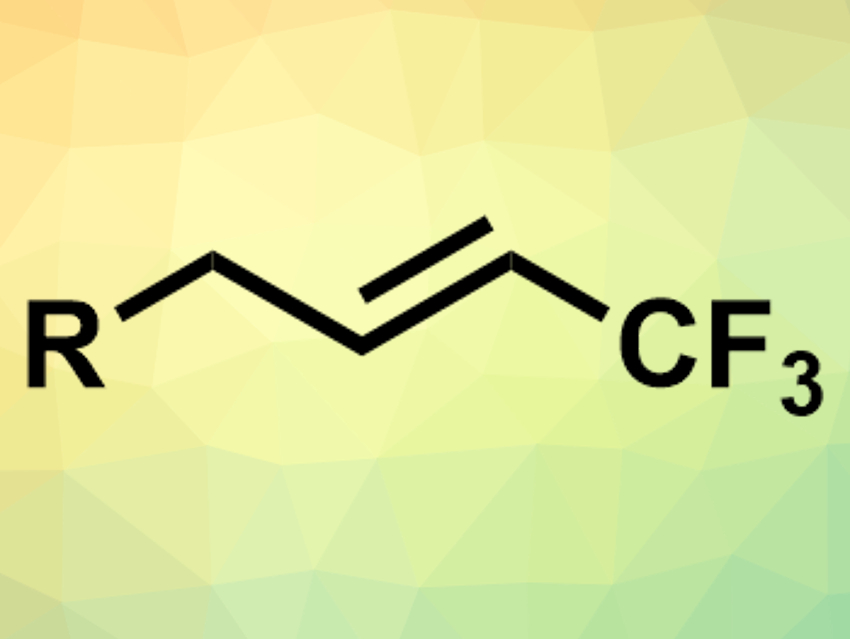
Trifluoromethyl groups are useful, e.g., in pharmaceutical chemistry. There is a range of methods for introducing them into organic compounds. However, the trifluoromethylation of unactivated alkenes is still challenging. The available methods either suffer from limited E/Z selectivity or they require metal-based photocatalysts, harsh oxidants, or hard-to-handle gaseous trifluoromethyl sources.
Xinkan Yang and Gavin Chit Tsui, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, have developed a protocol for the trifluoromethylation of unactivated alkenes that proceeds under mild conditions, without a photocatalyst, and with high E-selectivity, and with an easy-to-handle liquid trifluoromethyl source (Me3SiCF3). The team reacted a wide range of alkenes with Me3SiCF3 under blue LED light in the presence of N-iodosuccinimide (NIS) as a mild oxidant and radical source, sodium acetate as an initiator, and silver acetate as an additive.
The desired E-alkenes were obtained in good yields. The reaction is milder, simpler, and more cost-effective than existing approaches.
- Trifluoromethylation of Unactivated Alkenes with Me3SiCF3 and N-Iodosuccinimide,
Xinkan Yang, Gavin Chit Tsui,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b00332