Carbon nitrides can be used as heterogeneous photocatalysts to promote organic reactions. Potassium poly(heptazine imide) (K-PHI), for example, is a member of the carbon nitride family and can efficiently catalyze different oxidation processes. One such reaction is the addition of aminium radicals (R2N∙) to double bonds. Examples of this type of reaction with C=O bonds, however, are rare.
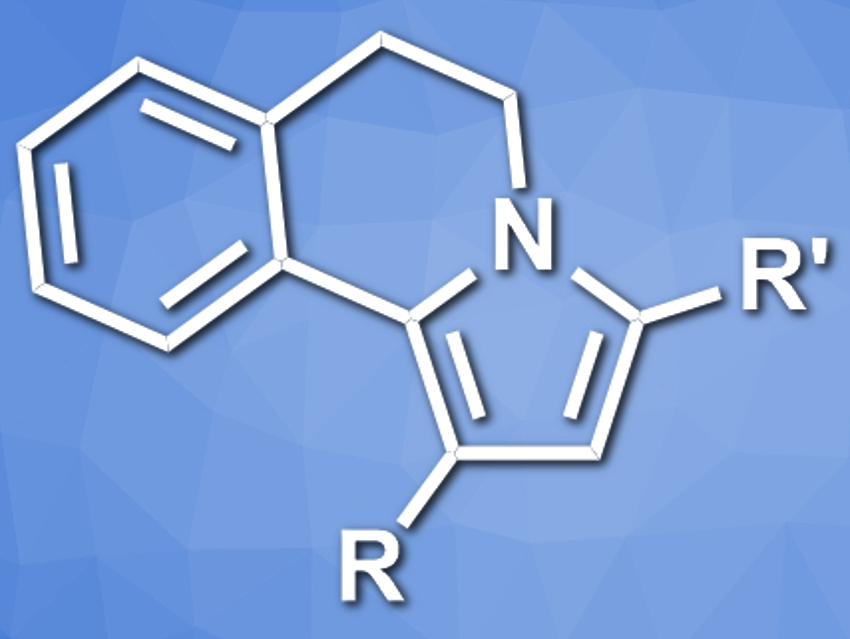
Aleksandr Savateev, Max-Planck Institute of Colloids and Interfaces, Potsdam, Germany, and colleagues have developed a photocatalytic reaction between tetrahydroisoquinoline and chalcones (a type of aromatic ketone) that gives N-fused pyrroles (pictured). The reaction uses K-PHI as a heterogeneous, recyclable photocatalyst that is active under visible light. Using tetrahydroisoquinoline (THIQ) derivatives as a source of aminium radicals, the team converted a range of chalcones to the corresponding N-fused pyrroles, i.e., 1,3-disubstituted-5,6-dihydropyrrolo[2,1-a]isoquinolines (DHPIQ), in the presence of K-PHI under a blue LED.
The reaction proceeds in good to excellent yields. According to the researchers, the C–N bond between THIQ and the carbon atom of the C=O group is formed by the coupling of a THIQ-derived radical and a chalcone-derived radical. The pyrrole ring is then formed via a Mannich-like cyclization. The products show strong fluorescence in the blue region with an internal quantum efficiency (IQE) of up to 24 %.
- Carbon nitride photocatalyzes regioselective aminium radical addition to the carbonyl bond and yields N-fused pyrroles,
Bogdan Kurpil, Katharina Otte, Artem Mishchenko, Paolo Lamagni, Wojciech Lipiński, Nina Lock, Markus Antonietti, Aleksandr Savateev,
Nat. Commun. 2019.
https://doi.org/10.1038/s41467-019-08652-w