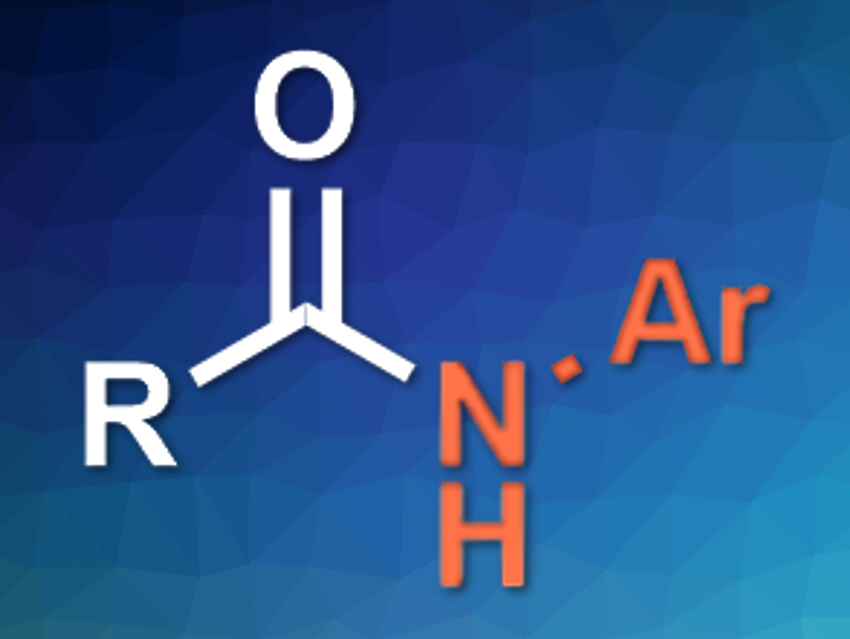
Amides are key components of many biologically active compounds and some materials. Usually, they are synthesized from amines or anilines using amidation reactions. The anilines used for these processes are often made from nitroarenes. Thus, directly using nitroarenes in amidation reactions would be a more step-economic alternative.
Meiming Luo, Sichuan University, Chengdu, China, Xiaoming Zeng, Sichuan University and Xi’an Jiaotong University, China, and colleagues have developed a protocol for the reductive amidation of esters with nitroarenes. The team used CrCl3 as a precatalyst, 4,4′-di-tert-butyl-2,2′-bipyridine (dtbpy) as a ligand, magnesium as a reductant, and chlorotrimethylsilane (TMSCl) as an additive. The esters were converted to the corresponding amides (pictured) in good yields.
The elemental magnesium and the chromium-based catalyst activate the acyl C–O bond of the ester via a single-electron reduction. Mg is also used to reduce the nitroarene. The reduced intermediates react with the activated acyl group to give the desired amides. Both the substrates and the precatalyst are inexpensive and the reaction is step-economic.
- Chromium-Catalyzed Activation of Acyl C–O Bonds with Magnesium for Amidation of Esters with Nitroarenes,
Liang Ling, Changpeng Chen, Meiming Luo, Xiaoming Zeng,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b00554