Enantioselective reactions often use chiral auxiliaries, i.e., chiral groups that are bound to the substrate during the reaction, ensure the desired selectivity, and are later removed. Chiral lithium amides can replace these covalently bound auxiliaries in some reactions. These compounds form aggregates with organometallic reagents and induce enantioselectivity in this way, without being covalently bound to the substrate. They can be considered noncovalent, or “traceless”, chiral auxiliaries.
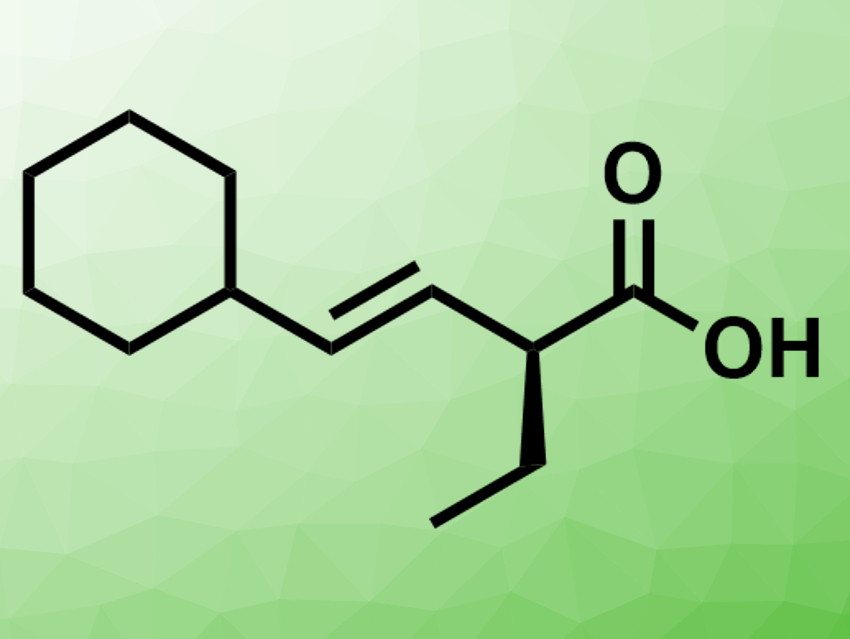
Armen Zakarian and colleagues, University of California, Santa Barbara, USA, have developed a protocol for the regioselective and enantioselective alkylation of β,γ-unsaturated carboxylic acids (example product pictured). The team used n-BuLi to convert β,γ-unsaturated carboxylic acids to lithium enediolate intermediates. 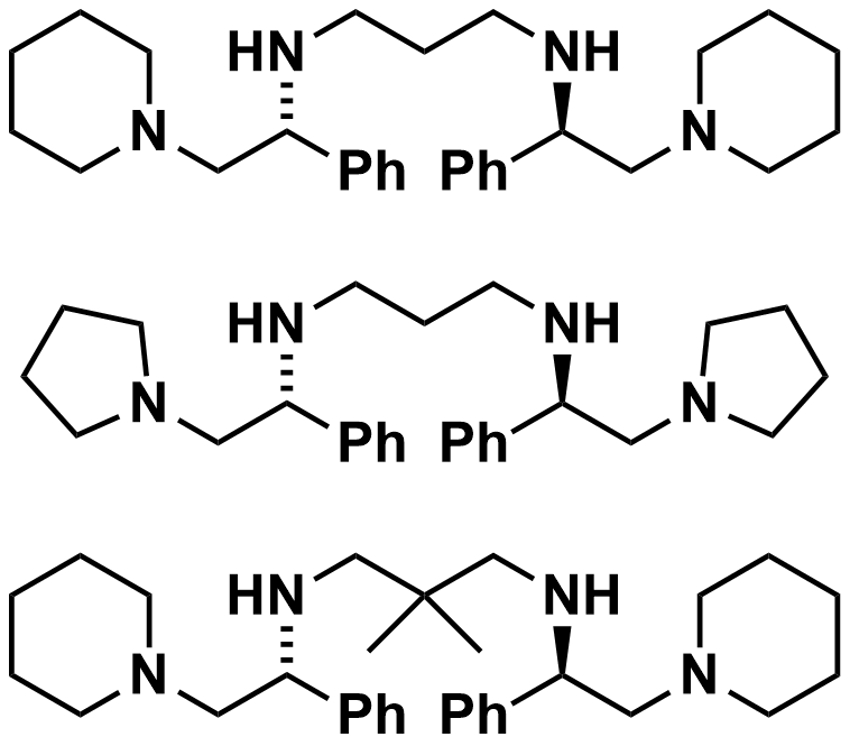 Chiral amines (examples pictured right) were then used as traceless auxiliaries for the addition of alkylating agents such as iodoethane. They form an aggregate with the lithium enediolates.
Chiral amines (examples pictured right) were then used as traceless auxiliaries for the addition of alkylating agents such as iodoethane. They form an aggregate with the lithium enediolates.
The reaction gives the desired alkylation products in good yields and with high regioselectivities and enantioselectivities. The chiral auxiliary can be recovered by a simple extraction and reused. The exact structure of the aggregate between the lithium enolate and the chiral lithium amide is unclear. According to the team, this structure will be investigated in the future.
- Direct Enantioselective and Regioselective Alkylation of β,γ-Unsaturated Carboxylic Acids with Chiral Lithium Amides as Traceless Auxiliaries,
Kai Yu, Bukeyan Miao, Wenqi Wang, Armen Zakarian,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b00587