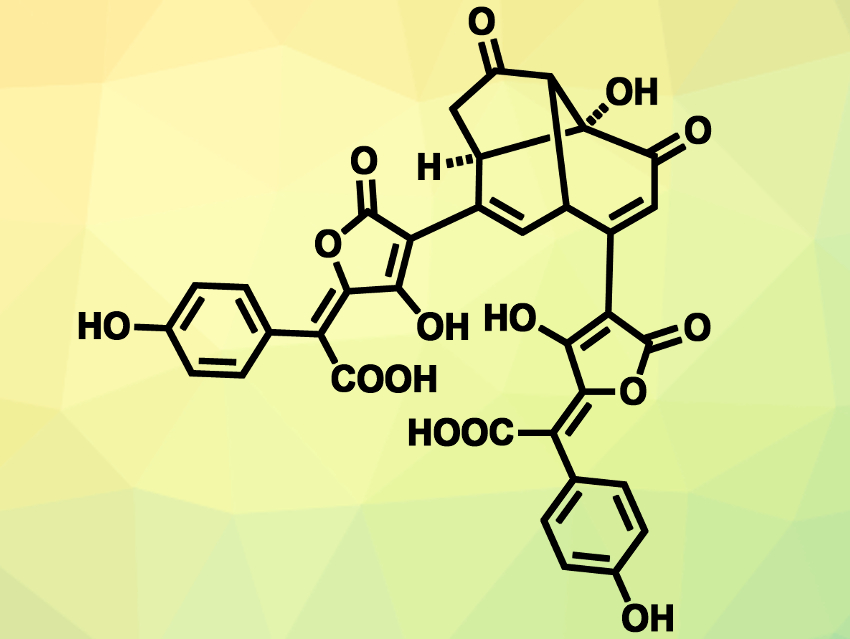
There is a series of natural products isolated from fungi that can be considered dimeric derivatives of xerocomic acid: norbadione A, badione A, sclerocitrin, and chalcitrin (pictured). This type of compound could serve as a complexation agent for metal ions in the fungi. Chalcitrin is a yellow compound found in the fungus Chalciporus piperatus or “peppery bolete”. It is the rarest of the substances mentioned above: Only 2 mg of the compound can be isolated from 300 g of fungus.
Scott A. Snyder and colleagues, University of Chicago, IL, USA, have performed the first total synthesis of chalcitrin. The team first built the polycyclic core of the compound (pictured top right), using an Au(I)-catalyzed Conia ene reaction and an acyloin addition as key steps. The two identical side chains were introduced in one step using a double Stille coupling. The overall synthesis involves 17 linear steps. The team obtained about 70 mg of the compound.
The researchers initially found that the NMR spectra of the product were not in agreement with the ones reported for the compound isolated from fungi. However, when the team added sodium methylsulfinylmethylide (NaDMSO) to convert the product to its sodium dicarboxylate salt, they obtained matching spectral data. This indicates that the previously isolated natural product was not the free acid, but a dicarboxylate salt.
- The Total Synthesis of Chalcitrin,
Ming Yang, Fangjie Yin, Haruka Fujino, Scott A. Snyder,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.8b12612