Cyclic compounds with conjugated bonds (such as benzene) are usually aromatic only in the ground state or only in their first excited state. Molecules with so-called adaptive aromaticity, which are aromatic in both of these electronic states, are very rare.
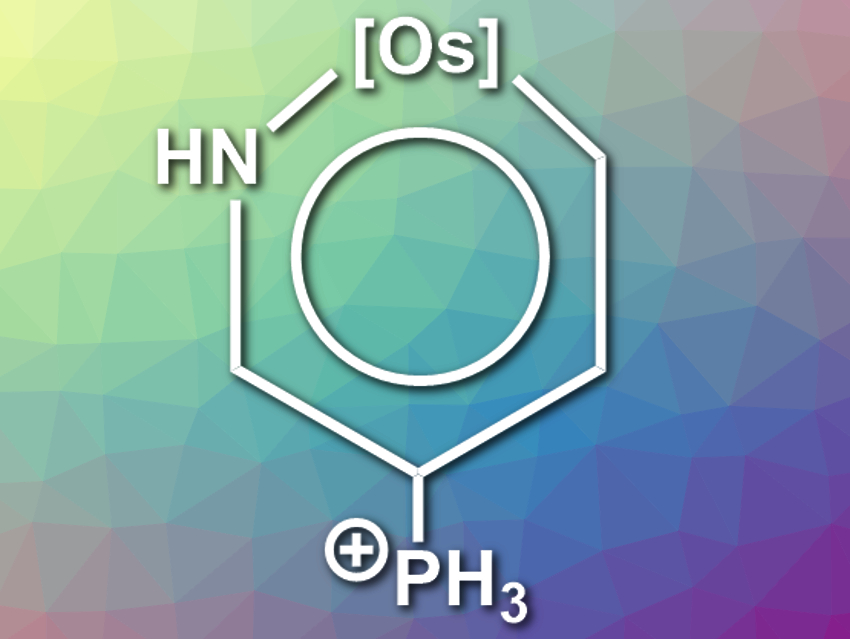
Jun Zhu and colleagues, Xiamen University, China, have found the first example of a compound that has a triplet ground state and shows dual aromaticity in both the lowest triplet and the lowest singlet state: an osmapyridinium with phosphonium substituents (pictured, [Os] = OsCl2(PH3)2). The team performed density functional theory (DFT) calculations of 96 different osmapyridine and osmapyridinium compounds. For the compounds calculated to have a planar structure, the researchers evaluated the aromaticity based on the proposed structure, calculated electronic properties, and heats of hydrogenation.
The team found one compound with adaptive aromaticity and a triplet ground state (pictured) and one compound with adaptive aromaticity and a singlet ground state, which is similar in structure, but has no phosphonium substituent. According to the researchers, such compounds could be useful in photochemistry and molecular magnetism applications.
- Dual Aromaticity in Both the T0 and S1 States: Osmapyridinium with Phosphonium Substituents,
Ting Shen, Dandan Chen, Lu Lin, Jun Zhu,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.8b11564