Mechanochemical methods use mechanical energy, such as compression, shear, or friction, for chemical transformations. They have great potential for use in solvent-free synthesis. The use of ball milling techniques has extended its application to the synthesis of architecturally complex targets. Many biologically building blocks, such as amino acids, peptides, saccharides, nucleotides, and nucleosides, have been prepared by mechanochemical synthesis. However, mechanosynthesis of lipids by ball milling techniques has remained essentially unexplored.
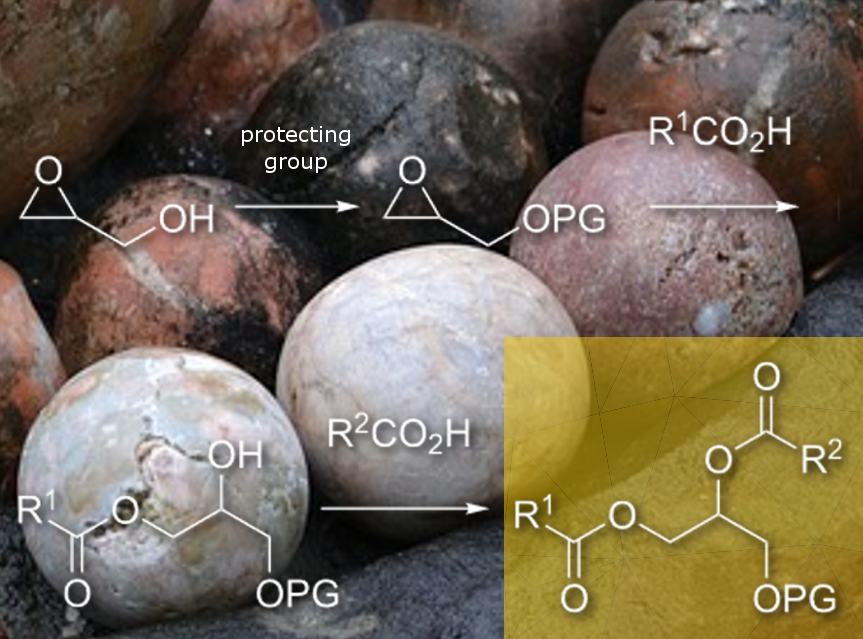
José G. Hernández and Carsten Bolm, RWTH Aachen University, Germany, and colleagues have developed a mechanochemical multistep synthetic route to mono- and diacylglycerol derivatives. The team started with glycidol (pictured) and added a hydroxy protecting group. This step is followed by a metal-catalyzed epoxide-ring opening and the solvent-free ester formation between monoacylglycerols and fatty acids to afford diacylglycerols (DAGs).
DAGs are glycerolipids containing two fatty acids esterified to the alcohol glycerol. They are important due to their signaling functions in cells. Biological routes to form DAGs include enzymatic degradation of glycerophospholipids and lipolysis of triacylglycerols (TAGs). However, due to the structural diversity of fatty acids present in acylglycerols and to the small structural differences among these fatty acids (e.g., chain length, degree of unsaturation, double bond position, stereochemistry), the access to pure DAGs and TAGs from natural sources by extraction is inconvenient. An alternative chemical synthesis starting from glycerol or glycidol involves multiple preparative steps in organic solvents, e.g., CH2Cl2, THF, Et2O.
- Synthesis of acylglycerol derivatives by mechanochemistry,
Karen J. Ardila-Fierro, Andrij Pich, Marc Spehr, José G. Hernández, Carsten Bolm,
Beilstein J. Org. Chem. 2019.
https://doi.org/10.3762/bjoc.15.78