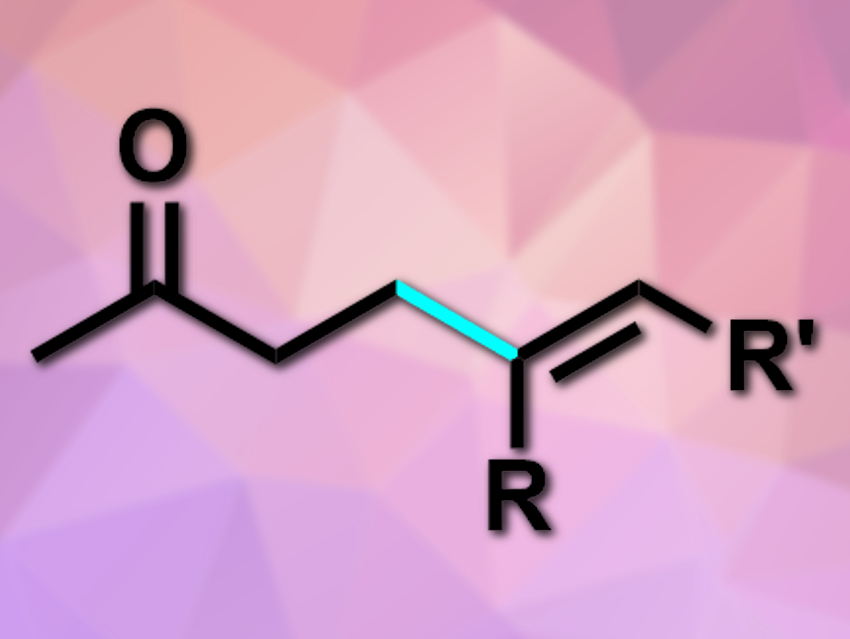
Carbonyl compounds are very commonly used in organic syntheses. They are most often used as carbonyl electrophiles and can also be functionalized at the acidic α-position. Their β-position, in contrast, is less reactive and more difficult to functionalize.
Guangbin Dong and colleagues, University of Chicago, IL, USA, have developed a direct β-alkenylation of ketones using alkenyl bromides. The team used linear or cyclic ketones and a wide variety of alkenyl bromides, such as 3-bromocoumarins, as substrates, Pd(MeCN)4(OTf)2 as a catalyst, P(iPr)3 as a ligand, potassium hydrogen phthalate as an additive, and acetonitrile as the solvent.
The desired products (example pictured) were obtained in moderate to good yields. The reaction has good functional group tolerance, is redox-neutral, and works without directing groups or strong acids or bases. According to the researchers, it proceeds via a Pd-catalyzed redox cascade. The products can be used as versatile substrates for further chemical transformations.
- Direct β-Alkenylation of Ketones via Pd-Catalyzed Redox Cascade,
Chengpeng Wang, Alexander J. Rago, Guangbin Dong,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b01116