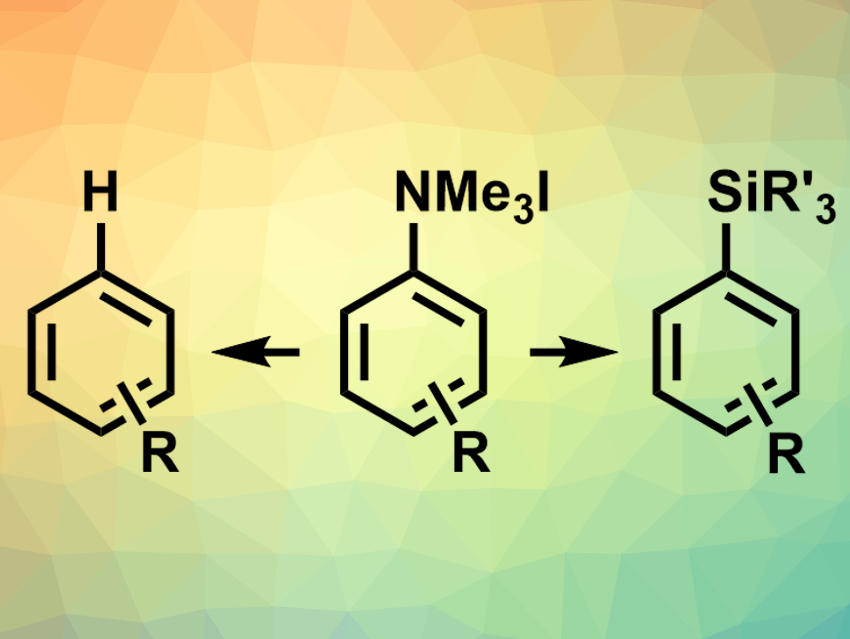
Aniline derivatives are widely available from chemical feedstocks. Aryl trialkylammonium salts, in particular, could be useful in synthesis, as they are easy to access and handle. There is a range of known conversions of these ammonium salts, but no direct conversion of aryl trialkylammonium salts to aryl silanes had been reported so far.
Alexander W. Rand and John Montgomery, University of Michigan, Ann Arbor, USA, have developed a method for the reduction of aryl ammonium salts to aryl silanes or arenes (pictured) using nickel catalysis. The reaction shows ligand-controlled selectivity. The team used Ni(COD)2 (COD = 1,5-cyclooctadiene) as a catalyst, an N-heterocyclic carbene (NHC) with isopropyl and methoxy substituents as a ligand, NaOtBu as a base, and dioxane as a solvent to convert aryl trimethylammonium iodides and silanes to aryl silanes (pictured right). The reaction has a broad substrate scope and provides moderate to excellent yields depending on the reactants.
When the NHC ligand was replaced with a less electron-rich, mesityl-substituted derivative, the selectivity of the reaction changed and the corresponding arene (pictured left) was generated from the ammonium salt in synthetically useful yields. According to the researchers, the method provides a new way to functionalize common C(sp2)–N bonds with nickel as an earth-abundant catalyst.
- Catalytic reduction of aryl trialkylammonium salts to aryl silanes and arenes,
Alexander W. Rand, John Montgomery,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc01083a