The Suzuki–Miyaura cross-coupling reaction is an important method for the formation of C–C bonds. There is a wide range of organoboron nucleophiles suitable for the reaction. The electrophiles are usually sulfonates or organohalides. Alternative electrophiles could be useful in cases where these compound classes are not reactive enough or difficult to access.
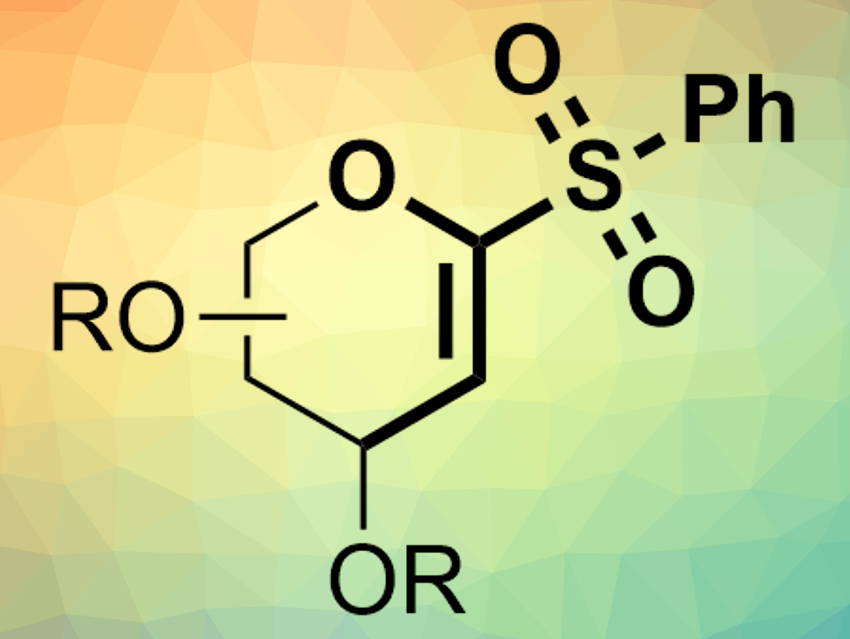
Dawen Niu and colleagues, Sichuan University, Chengdu, China, have shown that α-oxo-vinylsulfones (example pictured) can be used as electrophiles in nickel-catalyzed Suzuki–Miyaura cross-coupling reactions. The team used cyclic or acyclic α-oxo-vinylsulfones as electrophiles, different boronic acids or boronic acid pinacol esters as nucleophiles, Ni(COD)2 (COD = 1,5-cyclooctadiene) as a catalyst together with phosphine ligands, KOH as a base, and tetrahydrofuran (THF) as a solvent.
The desired products, e.g., C-aryl glycals or acyclic vinyl ethers, were obtained in high yields. The reaction has a good functional group tolerance and proceeds under mild conditions (60 °C). According to the researchers, the α-heteroatom in the vinylsulfone electrophiles is critical for the reaction.
- Ni-Catalyzed Suzuki–Miyaura Cross-Coupling of α-Oxo-vinylsulfones To Prepare C-Aryl Glycals and Acyclic Vinyl Ethers,
Liang Gong, Hong-Bao Sun, Li-Fan Deng, Xia Zhang, Jie Liu, Shengyong Yang, Dawen Niu,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b02312