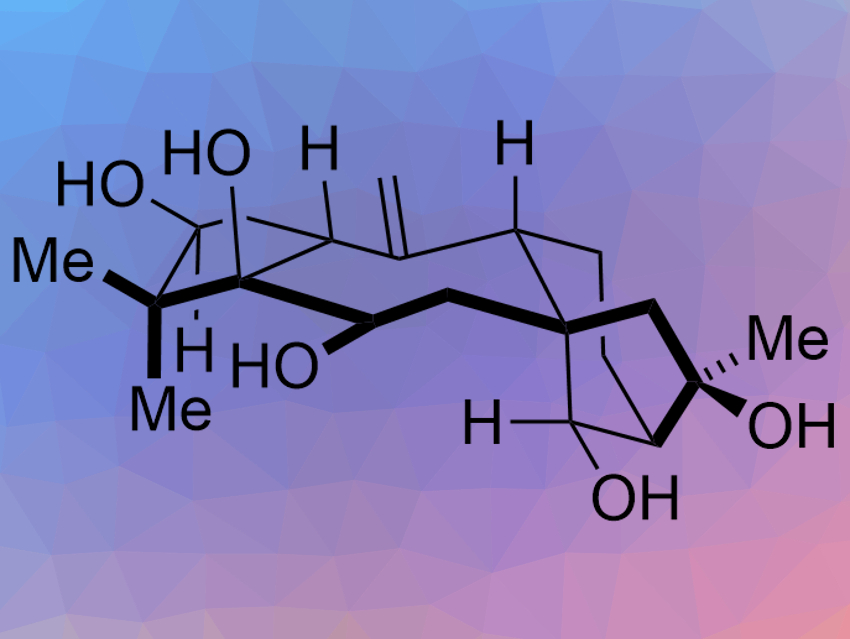
Grayanane diterpenoids are complex, multicyclic natural products with potential uses in pharmaceutical chemistry. Grayananes are a type of rearranged kaurane, a large class of natural products. Only a few of the over 1,000 isolated kaurane derivatives have been synthesized.
Timothy R. Newhouse and colleagues, Yale University, New Haven, CT, USA, have performed the first total synthesis of the grayanane principinol D (pictured). The team used a convergent fragment-coupling approach that allows them to build the central ring last and preserve all the “outer” stereocenters. The fragments, a cyclopentyl fragment and a bicyclo[3.2.1]octane fragment, were first connected by a 1,2-addition reaction. An SmI2-catalyzed reductive cyclization was then used to close the central seven-membered ring.
The total synthesis involves 19 steps overall. The NMR spectra of the product are in agreement with those reported for the isolated natural product. According to the researchers, the fragment-based approach should allow the synthesis of a range of grayanane derivatives, which could then be tested for their biological activities.
- Convergent Total Synthesis of Principinol D, a Rearranged Kaurane Diterpenoid,
Aneta Turlik, Yifeng Chen, Anthony C. Scruse, Timothy R. Newhouse,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b03751