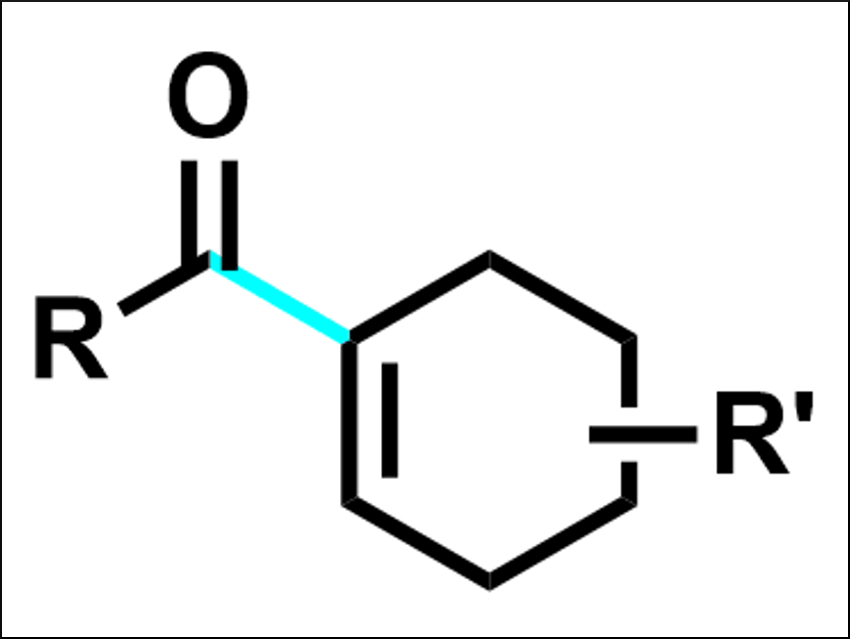
Enones are found in many biologically active products. They also are useful in synthetic organic chemistry. enones can, for example, be synthesized by cross-coupling reactions between vinyl and acyl fragments, usually acyl electrophiles and vinyl nucleophiles. There are also cross-couplings between two electrophiles. However, there had been no method for the reductive acylation of vinyl electrophiles to give enones so far.
Feng-Feng Pan, Peng Guo, Chun-Ling Li, Peifeng Su, and Xing-Zhong Shu and colleagues, Lanzhou University, China, have developed such a reaction, catalyzed by nickel species. The team used Ni(dppe)Cl2 as a catalyst (dppe = 1,2-bis(diphenylphosphino)ethane), 4,4′,4″-tri-tert-butyl-2,2′:6′,2″-terpyridine as a ligand, Mn as a reducing agent, and a mixture of dimethylacetamide (DMA) and toluene as the solvent. The reaction between different cyclic or acyclic vinyl triflates and a broad range of acid fluorides as acyl reactants was performed at 70 °C.
The desired enones (example pictured) were obtained in moderate to good yields. According to the researchers, the reaction is the first cross-electrophile reaction of acid fluorides as well as the first reductive acylation of vinyl electrophiles. The method could be useful for the late-stage functionalization of pharmaceuticals.
- Enones from Acid Fluorides and Vinyl Triflates by Reductive Nickel Catalysis,
Feng-Feng Pan, Peng Guo, Chun-Ling Li, Peifeng Su, Xing-Zhong Shu,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b01164