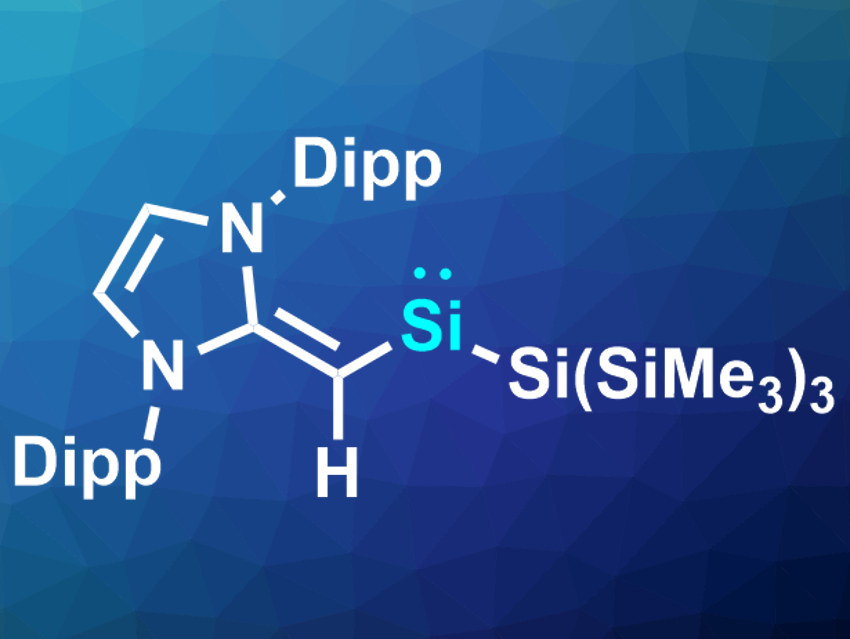
Tetrelenes (ER2, E = Si, Ge, Sn, Pb) are divalent main group compounds. They are the inorganic equivalents of carbenes and are usually very unstable. Such compounds can often activate small molecules, e.g., H2 or CO2. However, due to their reactivity, they can be difficult to synthesize and stabilize. For example, there are only a few known examples of isolable two-coordinate acyclic silylenes (R2Si) so far.
Eric Rivard and colleagues, University of Alberta, Edmonton, Canada, have synthesized another member of this rare class of compounds: (MeIPrCH)Si{Si(SiMe3)3} (pictured, Dipp = 2,6-iPr2C6H3). The team started the synthesis from the bulky vinyl ligand, which was reacted with SiBr4. The resulting tribromo-vinylsilane was reduced with hypersilyl potassium [K(THF)2][Si(SiMe3)3] (THF = tetrahydrofuran) to give the desired silylene. According to the researchers, is the first example of such a compound supported by a carbon-based ligand.
The silylene is stable at room temperature under a nitrogen atmosphere. It was characterized using X-ray crystallography and 1H and 29Si NMR spectroscopy. The team also investigated the reactivity of the compound and found that, despite its relative stability, it can activate bonds such as H–B, P–P, or C–O bonds.
- A Vinyl Silylsilylene and its Activation of Strong Homo- and Heteroatomic Bonds,
Matthew M. D. Roy, Michael J Ferguson, Robert McDonald, Yuqiao Zhou, Eric Rivard,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc01192g