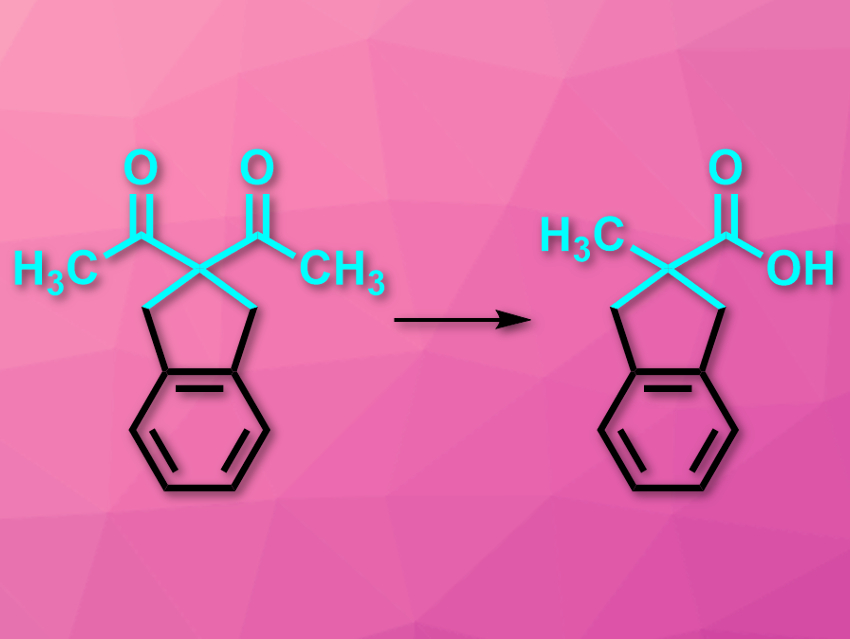
Carboxylic acids next to quarternary all-carbon centers can be difficult to make due to the high steric congestion. The synthesis of such compounds usually requires nitrile anions, strong nucleophiles, and water-free conditions. The oxidative rearrangement of 1,3-diketones (pictured) could be a milder alternative to these reactions. However, this approach has been rarely used because of its dependence on the substrate’s structure and a lack of control over the regioselectivity.
Abraham Mendoza, Stockholm University, Sweden, and colleagues have studied the mechanism of the reaction and found that it can be used in a mild, efficient, and practical protocol in basic media. The team used a symmetric substrate with a cyclic substituent (pictured) as a model and added H2O2 to initiate the oxidative rearrangement. The reaction was monitored using NMR spectroscopy.
The researchers tested commonly used additives such as acetic acid and found that they actually inhibit the reaction instead of promoting it. Basic additives such as LiOH, in contrast, led to higher conversion. The team also evaluated the regioselectivity of the reaction using asymmetric substrates. They found that the selectivities up to about 30:1 can be achieved by the rational design of the leaving group.
- Mechanism and regioselectivity of the anionic oxidative rearrangement of 1,3-diketones towards all-carbon quaternary carboxylates,
Emma Bratt, Samuel Suárez-Pantiga, Magnus J. Johansson, Abraham Mendoza,
Chem. Commun. 2019.
https://doi.org/10.1039/c9cc03331a