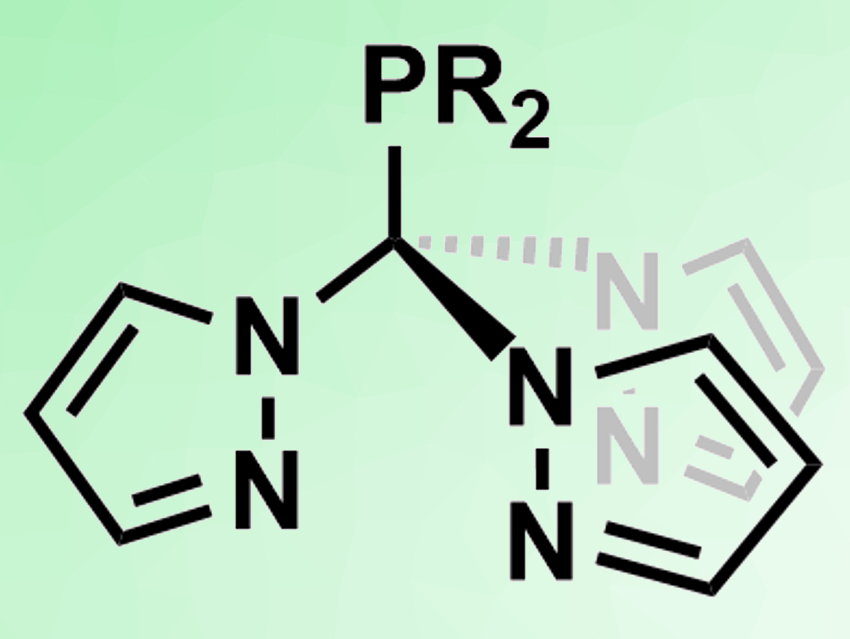
Scorpionate ligands are three-dentate ligands such as tris(pyrazolyl)hydroborates or tris(pyrazolyl)methanes. They usually coordinate to metals in a fac manner, i.e., at three adjacent positions. The ligands can be further functionalized by including a coordinating atom at the boron or carbon backbone.
Frank Breher and colleagues, Karlsruhe Institute of Technology (KIT), Germany, have synthesized a new type of scorpionate ligands, phosphine-functionalized tris(pyrazolyl)methane derivatives (pictured). The team reacted freshly prepared lithium tris(pyrazolyl)methanide with R2PCl (R = Ph, n-Bu, or i-Pr) to obtain the desired products.
The researchers prepared Rh, Ru, Pd, and Cu complexes with the prepared ligands and found that they can provide flexible coordination, with mixed N,P-heterochelate complexes, N,N,P-heteroscorpionate complexes, or purely P-coordinated complexes as products. The team also obtained a heterobimetallic complex containing palladium and copper, in which one tris(pyrazolyl)methyl phosphine ligand binds two metals.
- Phosphine-functionalised Tris(pyrazolyl)methane Ligands and their Mono- and Heterobimetallic Complexes,
Hanna E Wagner, Silvia Hohnstein, Max G. Schußmann, Lukas A. Steppe, Frank Breher,
Dalton Trans. 2019.
https://doi.org/10.1039/c9dt02057h