Alkyl aryl ethers are found in many pharmaceutically active molecules. Their preparation usually involves harsh conditions, which limits functional group tolerance, or expensive transition-metal catalysts. Nickel catalysis could be a greener low-cost alternative, but usually requires stoichiometric oxidants. The need for these oxidants could be avoided by combining nickel catalysis with photoredox catalysis. This, however, comes with another problem: photoredox catalysts can be expensive and/or toxic.
Bartholomäus Pieber, Max Planck Institute of Colloids and Interfaces, Potsdam, Germany, and colleagues have used a carbon nitride (CN) material as a photoredox catalysts in combination with a nickel complex to promote cross-couplings of aryl bromides with alcohols. The carbon nitride material was synthesized by the polymerization of urea and oxamide. The nickel complex was prepared from Br2·3H2O and di-tert-butylbipyridyl (dtbbpy).
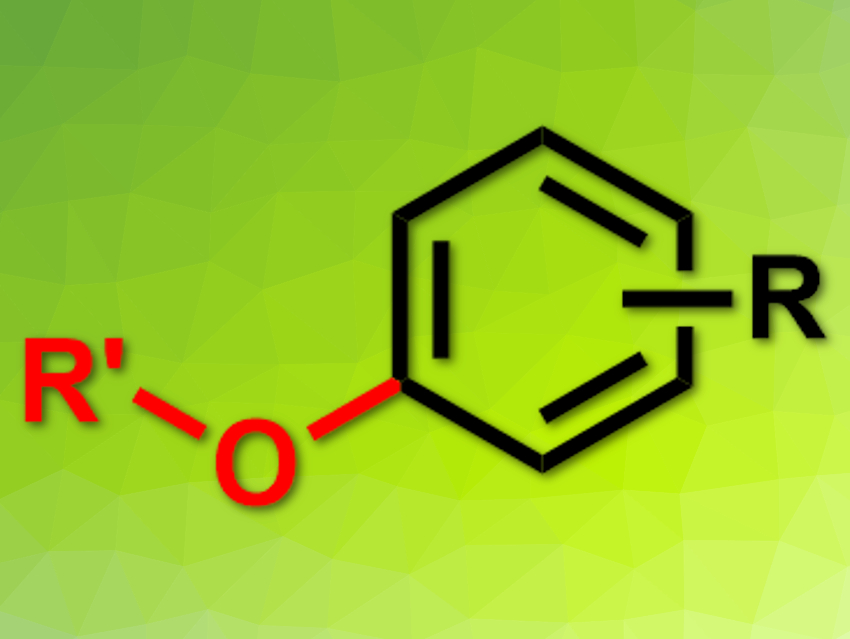
A variety of aryl bromides were coupled with a range of alcohols to give the desired ethers (pictured) in moderate to excellent yields. The reaction tolerates functional groups such as esters, nitriles, carbonyl groups, chlorides, trifluoromethyl groups, or methylsulfonyl groups. The combined catalyst can also be used to couple aryl iodides with thiols to give alkyl aryl thioethers.
- Semiheterogeneous Dual Nickel/Photocatalytic (Thio)etherification Using Carbon Nitrides,
Cristian Cavedon, Amiera Madani, Peter H. Seeberger, Bartholomäus Pieber,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b01957