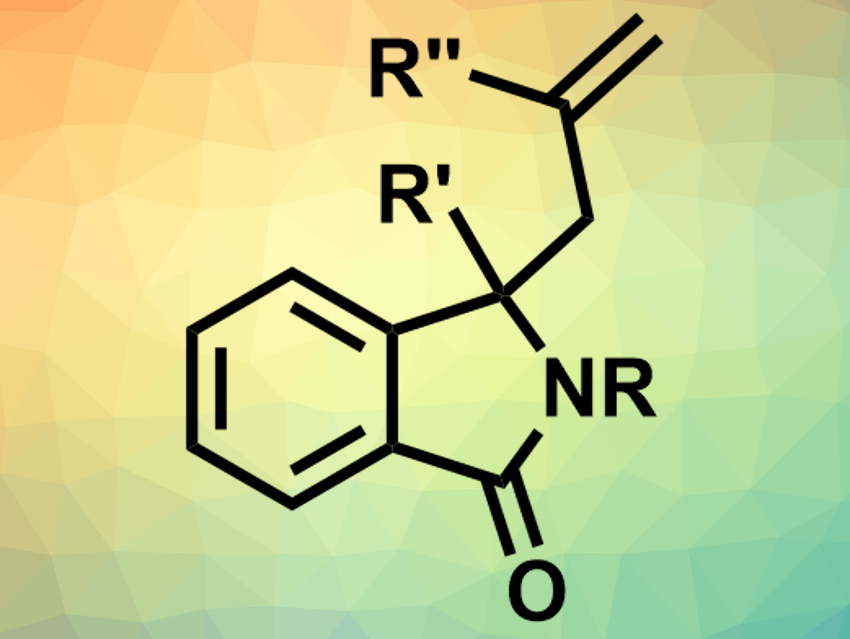
Isoindolinones are often found in natural products or drugs. This makes them interesting for use in medicinal chemistry. In order to access a great variety of isoindolinones, synthetic approaches that leave functional groups unaffected are useful.
Mark G. McLaughlin and Ashley J. Basson, Manchester Metropolitan University, UK, have developed a calcium-catalyzed Hosomi-Sakurai reaction for the allylation of isoindolinones. Different combinations of substituted isoindolinones and allyltrimethylsilane or branched allyl silanes were reacted to give the allylated products. The team used 1 mol% calcium(II) bis(trifluoromethanesulfonimide) (Ca(NTf2)2) as a catalyst, tetra-n-butylammonium hexafluorophosphate (nBu4NPF6) as an additive and 1,2-dichloroethane as the solvent and achieved yields up to 98 %.
According to the researchers, the reaction tolerates functional groups with both electron-donating and electron-withdrawing properties, as well as substituted phenyls and heterocycles. Some of the synthesized compounds were used for Suzuki cross-coupling and ring-closing metathesis reactions to prove the synthetic applicability of the allylated isoindolinones.
- Functionalisation of isoindolinones via a calcium catalysed Hosomi–Sakurai allylation,
Ashley J. Basson, Mark G. McLaughlin,
Chem. Commun. 2019.
https://doi.org/10.1039/c9cc02450f