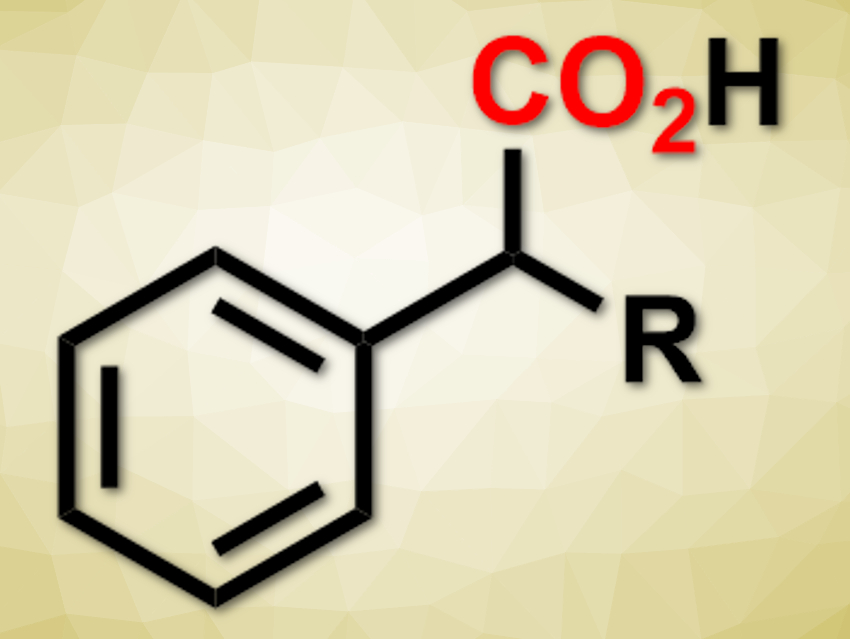
The greenhouse gas CO2 could be used as a sustainable chemical feedstock. It can, for example, be used to convert organic compounds into carboxylic acid derivatives. However, the direct carboxylation of C(sp3)–H bonds is still challenging.
Burkhard König, University of Regensburg, Germany, and colleagues have developed a visible-light-mediated carboxylation of benzylic C–H bonds with CO2 to give 2-arylpropionic acids (pictured). The team used 2,4,5,6-tetra(carbazol-9-yl)isophthalonitrile (4CzIPN) as a photosensitizer, triisopropylsilanethiol as a hydrogen atom transfer catalyst, and N,N-dimethylformamid (DMF) as the solvent. They converted a variety of compounds with benzylic C–H bonds into the corresponding carboxylic acids under visible light in moderate to excellent yields.
The proposed mechanism involves a single-electron oxidation of triisopropylsilanethiol, which produces a radical. This radical abstracts hydrogen from the substrate, giving a benzyl radical. The benzyl radical can then be reduced to give a carbanion, which reacts with CO2. The overall reaction is metal-free, atom-economic, and can, e.g., be used to synthesize commonly used drugs.
- Photocarboxylation of Benzylic C–H Bonds,
Qing-Yuan Meng, Tobias E. Schirmer, Anna Lucia Berger, Karsten Donabauer, Burkhard König,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b05360