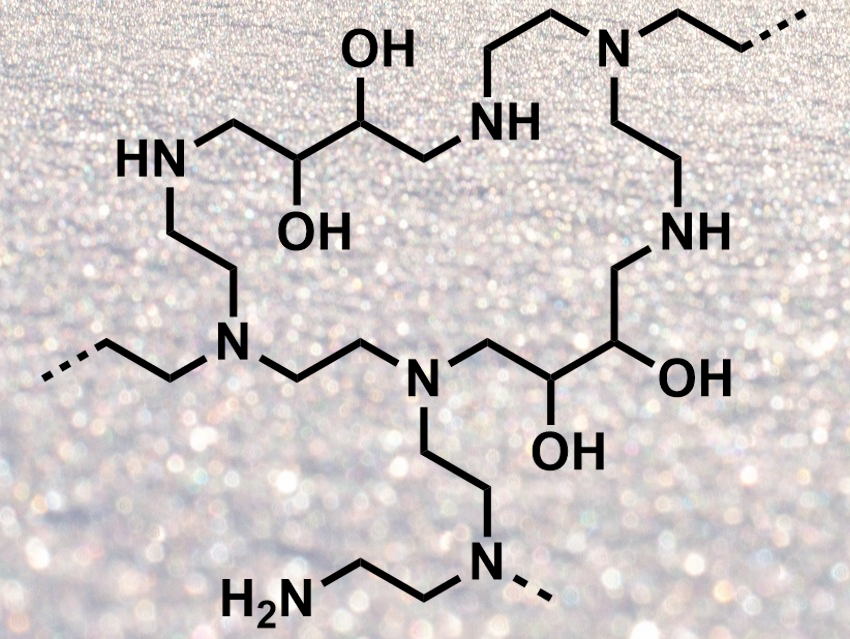
Amine-functionalized solid adsorbents can be used to capture the greenhouse gas CO2. Polyethylenimine (PEI) loaded onto a porous support is a promising example of this type of adsorbent. However, such adsorbents are often difficult to synthesize. Under humid conditions, they can lose some of the PEI due to leaching and do not absorb CO2 as well.
Xingguang Xu, Commonwealth Scientific Industrial Research Organisation (CSIRO), Perth, Australia, Colin D. Wood, CSIRO and Curtin University of Science and Technology, Perth, Australia, and colleagues have developed a new form of PEI which is easy to synthesize, does not need a support, and has a high CO2 uptake. The team prepared an aqueous solution of PEI and added 1,3-butadiene diepoxide (BDDE) as a crosslinker. This gave a PEI gel (structure pictured), which was ground to give a powdered, virtually dry PEI “snow”. The preparation of the adsorbent only takes 15 minutes.
The crosslinking avoids the leaching of PEI. The powder has a high surface area and, thus, a high CO2 capacity. The material’s performance is not significantly diminished by humidity. Adsorption columns using this material could potentially be a lot smaller than conventional ones due to the lack of a support material. According to the researchers, PEI “snow” could be a promising platform for industrial CO2 capture in the future.
- Polyethylenimine “Snow”: An Emerging Material for Efficient Carbon Removal,
Xingguang Xu, Bobby Pejcic, Charles Heath, Matthew B. Myers, Cara Doherty, Yesim Gozukara, Colin D. Wood,
ACS Appl. Mater. Interfaces 2019.
https://doi.org/10.1021/acsami.9b05921