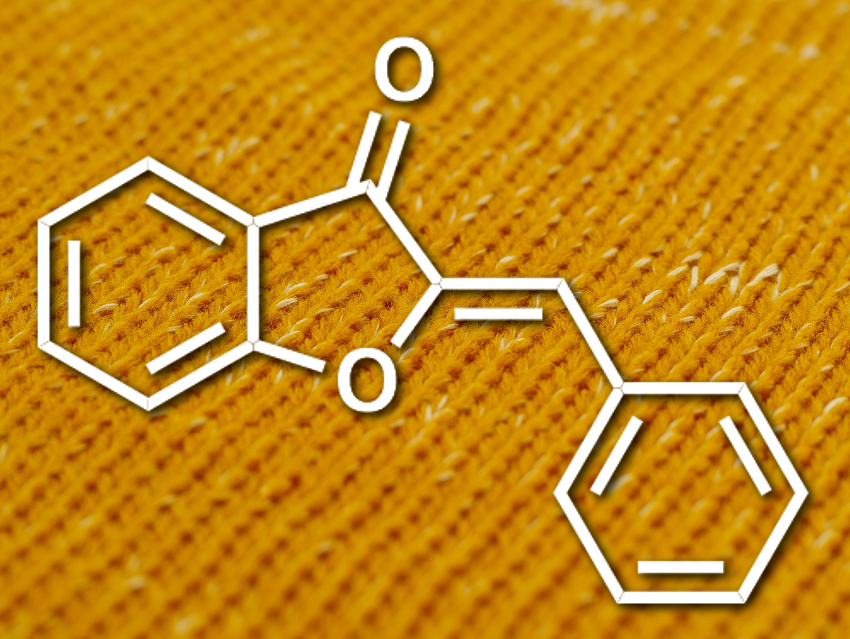
Aurones (pictured) are a subtype of the flavonoids, a family of natural products. They are named for their golden yellow color (aurum = Latin for gold). The compounds consist of a benzofuranone and a benzylidene group. While the properties of many other flavonoids are well studied, the aurones have garnered much less research interest. Most studies have focused on their fluorescence properties and on the influence of substituents on the benzylidene part. The use of aurones as dyes or pigments had not been reported so far.
Joza Schmitt and Scott T. Handy, Middle Tennessee State University, Murfreesboro, USA, have studied the influence of substituents at the benzofuranone ring on the optical properties of aurones, evaluated their toxicity, and tested the compounds’ potential as fabric dyes. The team synthesized a range of aurones from substituted benzofuranones and p-tolualdehyde via a Knoevenagel condensation.
The researchers recorded the UV–Vis spectra of the products to evaluate the influence of the different substituents. They found that the position of the substituent was generally more important than its type. Different substituents at the 4- or 5-positions of the benzofuranones caused a redshift of the absorption maximum by ca. 10 nm compared to the unsubstituted compound, while hydroxylation at the 6-position caused a significant blueshift of about 40 nm.
The team also evaluated the toxicity of the compound and found that, depending on the substitution, it was similar to slightly higher than the toxicity of the commonly used yellow dye tartrazine. Halogen substituents in the 6- or 7-positions of the benzofuranone led to lower toxicity. The researchers tested some of the synthesized aurones as fabric dyes and found that the compounds adhered to different natural fabrics such as silk and wool. Unexpectedly, they also gave vibrant colors on polypropylene, which can usually only be dyed efficiently when it is melted.
- A golden opportunity: benzofuranone modifications of aurones and their influence on optical properties, toxicity, and potential as dyes,
Joza Schmitt, Scott T. Handy,
Beilstein J. Org. Chem. 2019, 15, 1781–1785.
https://doi.org/10.3762/bjoc.15.171



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)