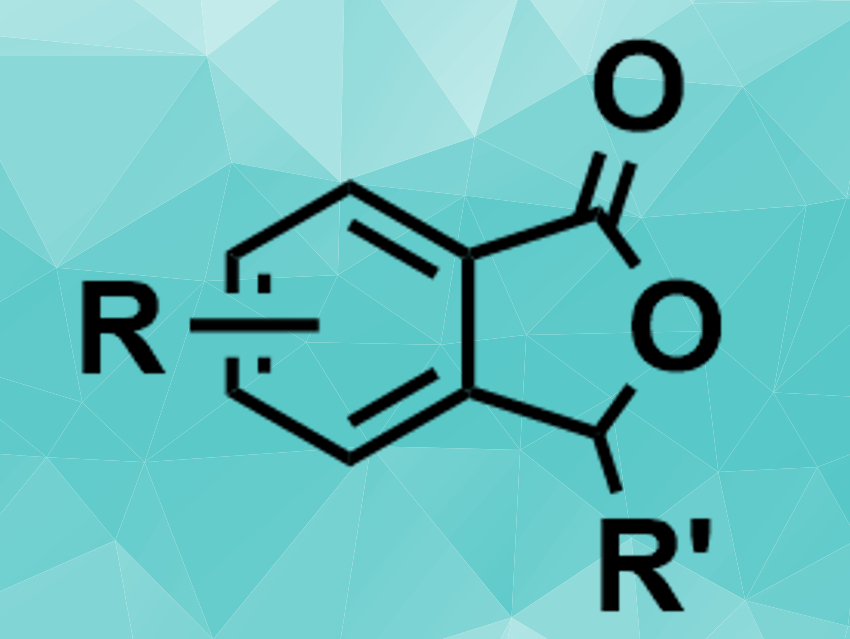
C–H bond activation reactions are useful in organic synthesis. However, the direct C–H addition to, e.g., aldehydes can be challenging. Benzamides, for example, are commonly used in C–H bond activation reactions, but only a few examples of this type of reaction between benzamides and aldehydes had been reported so far.
Guoliang Mao, Northeast Petroleum University, Daqing, China, Congyang Wang, Chinese Academy of Sciences and Huairou National Comprehensive Science Center, both Beijing, China, and colleagues have developed a rhenium-catalyzed C–H activation that allows the [4 + 1] annulation of benzamides and aldehydes to give phthalide derivatives (pictured). The team used different aromatic aldehydes and a range of substituted benzamides as substrates, ReBr(CO)5 as the catalyst, Me2Zn and ZnBr2 as additives, and 1,2-dimethoxyethane (DME) as the solvent.
The desired phthalide derivatives were obtained in moderate to good yields. The starting materials are readily available and the reaction tolerates both electron-donating and electron-withdrawing functional groups. According to the researchers, the method complements existing methods for phthalide synthesis.
- Rhenium-Catalyzed Phthalide Synthesis from Benzamides and Aldehydes via C–H Bond Activation,
Bing Jia, Yunhui Yang, Xiqing Jin, Guoliang Mao, Congyang Wang,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b02142