Thorium is an actinide commonly found in the +IV oxidation state. So far, only ten complexes in the rarer Th(III) oxidation state had been crystallographically characterized, most with cyclopentadienyl ligands. Examples of Th(IV) alkoxide and aryloxide complexes are known, while no Th(III) alkoxides or aryloxides had been reported.
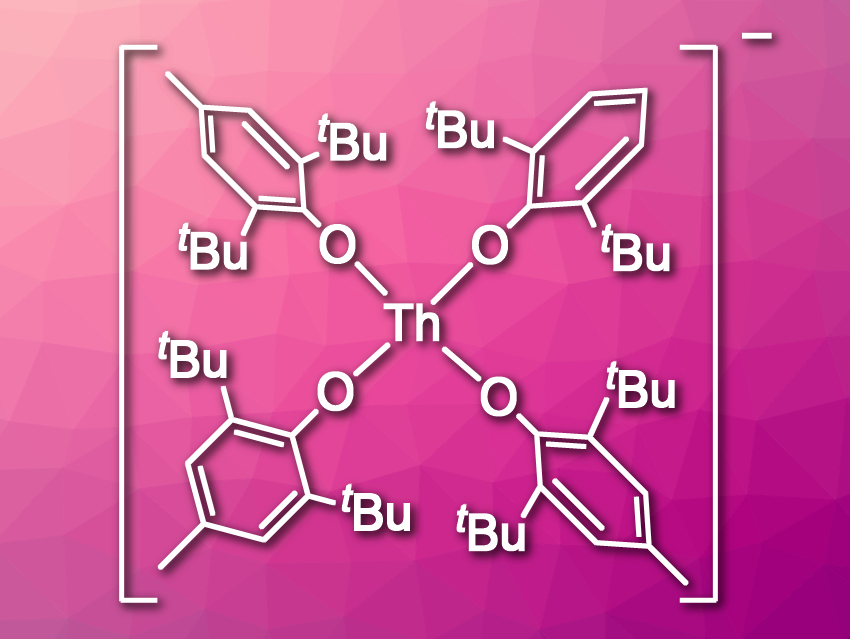
Filipp Furche, William J. Evans, and colleagues, University of California, Irvine, USA, have synthesized the first Th(III) aryloxide complex, [Th(OC6H2tBu2-2,6-Me-4)4]1– (pictured). They reduced the Th(IV) compound Th(OC6H2tBu2-2,6-Me-4)4 using either KC8 or Li in tetrahydrofuran (THF) and obtained the salts [K(THF)5(Et2O)][Th(OC6H2tBu2-2,6-Me-4)4] and [Li(THF)4][Th(OC6H2tBu2-2,6-Me-4)4]. The products were characterized using X-ray crystallography. The team found that the complex anions have a square-planar structure. According to the researchers, this is the first example of this complex structure in f-element chemistry.
The structure of this complex is surprising because a tetrahedral arrangement of the ligands should be sterically favored. The team suspects that the square-planar complex has an improved ligand field stabilization energy compared with a tetrahedral structure. In addition, the positioning of the eight tert-butyl substituents on the ligands is likely more favorable in the planar complex.
- Isolation of a Square-Planar Th(III) Complex: Synthesis and Structure of [Th(OC6H2tBu2-2,6-Me-4)4]1–,
Daniel N. Huh, Saswata Roy, Joseph W. Ziller, Filipp Furche, William J. Evans,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b04399