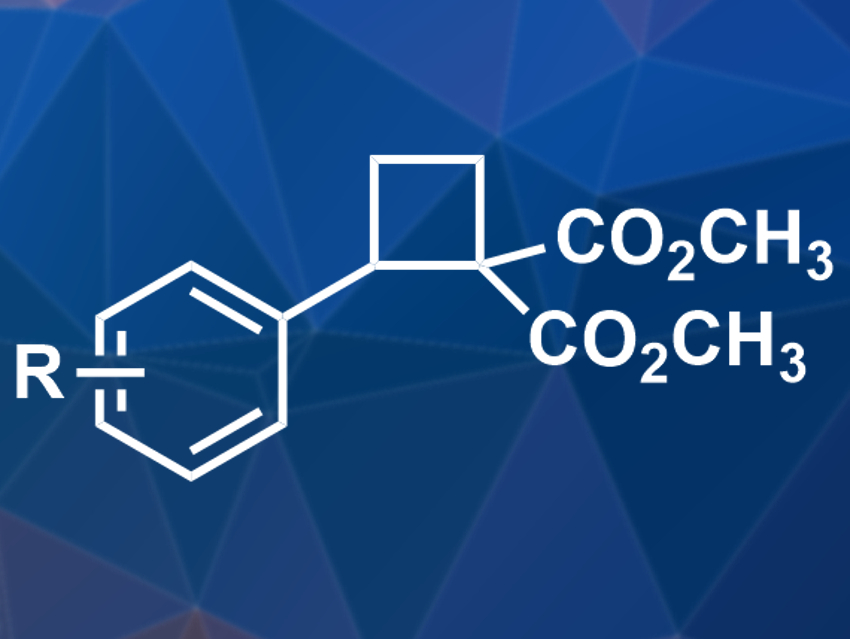
Ring strain, e.g., in cyclopropanes or cyclobutanes, can be used to drive chemical reactions. Unsubstituted cyclobutane is unreactive due to kinetic reasons, but substituted cyclobutanes can take part in reactions.
Daniel B. Werz and colleagues, Technische Universität Braunschweig, Germany, have developed a Friedel–Crafts-type reaction between electron-rich arenes and cyclobutanes with two geminal ester groups (pictured). The team used inexpensive AlCl3 as a catalyst and CH2Cl2 as the solvent. The reaction was performed at 0 °C and gave ring-opened products in moderate to very good yields. A broad variety of electron-rich arenes can be used as nucleophiles in this reaction.
The researchers found that other nucleophiles can also be used under the same conditions. Reactions with thiols or selenols also result in ring-opened products, in this case, γ-heteroatom-substituted diesters. These products were obtained in good yields.
- Ring-Opening Reactions of Donor–Acceptor Cyclobutanes with Electron-Rich Arenes, Thiols, and Selenols,
Alexander Kreft, Stephanie Ehlers, Peter G. Jones, Daniel B. Werz,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b02197