Polycyclic aromatic hydrocarbons (PAHs) can be useful, e.g., in organic electronic components. One compound of this type is truxene, a heptacyclic structure with threefold rotational symmetry. Truxenes have a central benzene core.
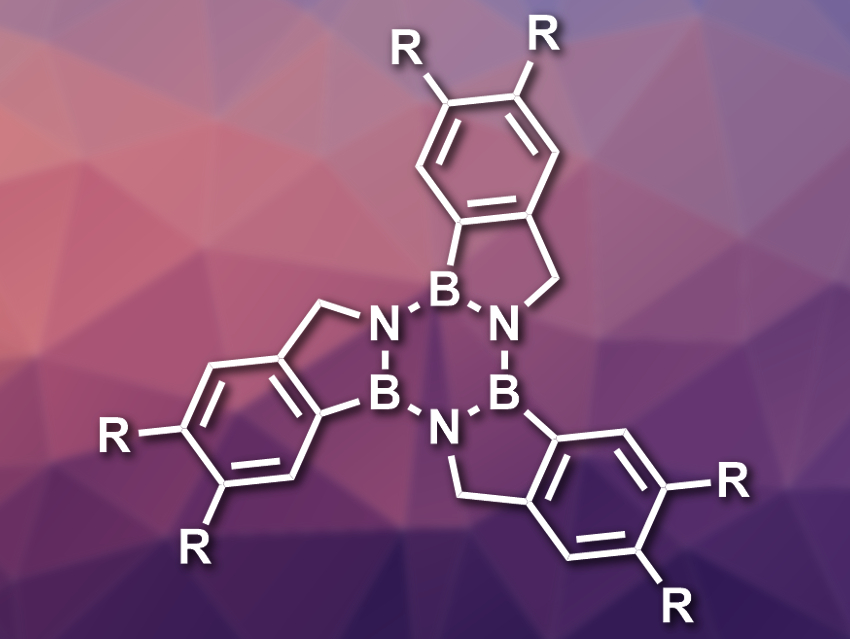
Dan Pantoş and colleagues, University of Bath, UK, have synthesized a series of substituted arene-borazine hybrids called borazatruxenes (pictured). In these compounds, the central benzene ring of truxene is replaced with a borazine ring (B3N3). This changes the electronic structure of the compounds significantly, while the structure remains very similar to the parent truxene.
The team started their synthesis from meta– and para-substituted 2-formylbenzeneboronic acids or 2-cyanobenzeneboronic esters, which were subjected to a ring-closing reaction to give amine-borane products (BN analogs of indane). Three equivalents of these bicyclic systems were combined under microwave irradiation to form the central borazine ring and give the desired products.
Usually, borazine-modified PAHs are moisture-sensitive, but the borazatruxenes are air- and moisture-stable. According to the researchers, the compounds could be useful in molecular electronic devices.
- Borazatruxenes,
Simone Limberti, Liam Emmett, Anamaria Trandafir, Gabriele Kociok-Köhn, G. Dan Pantoș,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc02489a