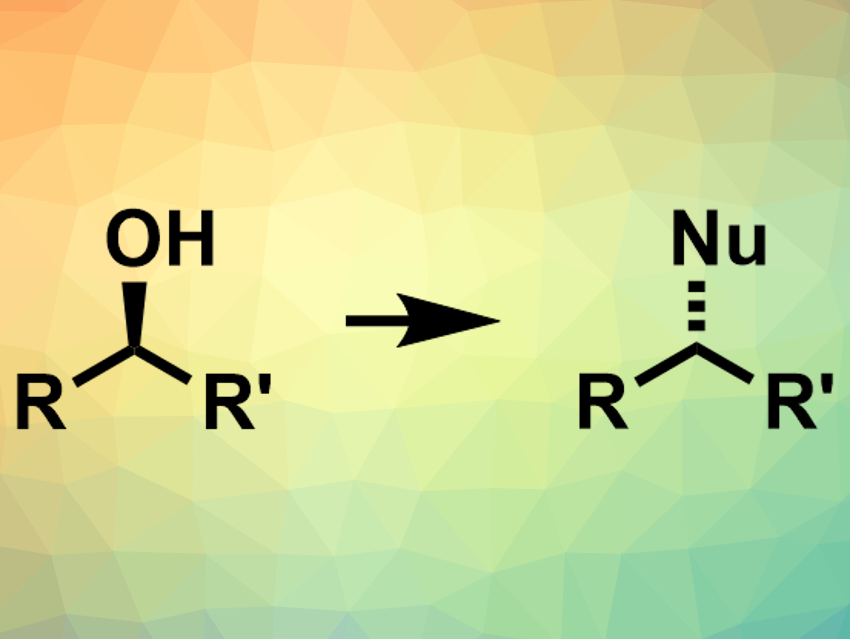
Alcohols are useful intermediates in organic chemistry. They can be converted to a variety of other functional groups using, e.g., nucleophilic substitution reactions (pictured). However, since OH– is not a good leaving group, these substitution reactions need additional reactants to activate the alcohol. Approaches like the commonly used Mitsunobu reaction produce stoichiometric byproducts that result from this activation.
In a traditional Mitsunobu reaction, triphenylphosphine (PPh3) is used as a stoichiometric reagent and is oxidized to triphenylphosphine oxide (Ph3P=O). There have been efforts to recycle these byproducts by reducing them to give back the original reagents, but this, again, requires stoichiometric amounts of redox reagents and is not atom-economical overall.
Ross M. Denton, University of Nottingham, UK, and colleagues have developed a redox-neutral catalytic variant of the Mitsunobu reaction. The team developed a phosphine-oxide-based catalyst that is active in the Mitsunobu reaction without changing the oxidation state at phosphorus. This catalyst, (2-hydroxybenzyl)diphenylphosphine oxide, can be synthesized in two-steps on a multigram scale.
Under the reaction conditions, the catalyst is dehydrated and cyclized to give an oxyphosphonium ion. This ion can react with the alcohol in a ring-opening reaction and activate the alcohol for the nucleophilic substitution. The desired product is formed and the catalyst is regenerated. Since there is no redox chemistry involved in the process, there is no need for stoichiometric reductants or oxidants and the process is atom-economical—with water as the only byproduct. According to the researchers, this approach could also be useful for a range of other phosphorus-mediated reactions.
- Redox-neutral organocatalytic Mitsunobu reactions,
Rhydian H. Beddoe, Keith G. Andrews, Valentin Magné, James D. Cuthbertson, Jan Saska, Andrew L. Shannon-Little, Stephen E. Shanahan, Helen F. Sneddon, Ross M. Denton,
Science 2019, 365, 910–914.
https://doi.org/10.1126/science.aax3353