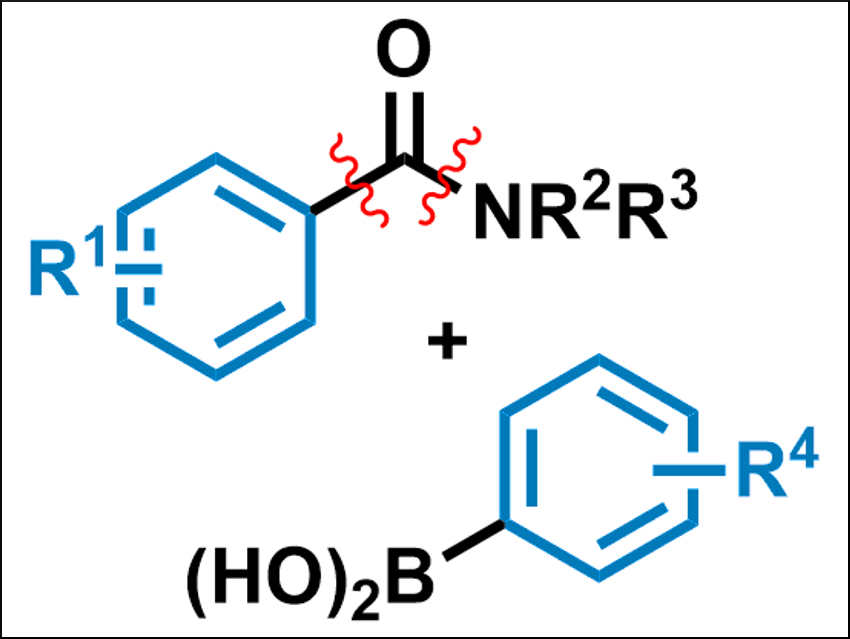
Palladium-catalyzed cross-coupling reactions, such as Suzuki-Miyaura reactions, are important tools in organic chemistry. Usually, aryl halides are used as electrophiles in Suzuki-Miyaura cross-couplings. However, alternative coupling partners such as amides could be useful to extend the scope of the reaction and to allow for selective functionalizations in complex molecules.
Michal Szostak, Rutgers University, Newark, NJ, USA, and colleagues have developed the first palladium-catalyzed decarbonylative Suzuki–Miyaura cross-coupling of amides to give biaryls (reactants pictured). The team used Pd(dppb)Cl2 (dppb = 1,4-bis(diphenylphosphino)butane) as the catalyst, NaHCO3 as a base, and dioxane as the solvent to couple a variety of aromatic amides with boronic acids at 160 °C.
The reaction proceeds via the selective activation of the amide’s carbon–nitrogen bond and proceeds under a loss of carbon monoxide. The desired biaryl products are obtained in high yields. According to the researchers, the method has the potential to enhance the utility of amides in cross-coupling reactions.
- Palladium-Catalyzed Decarbonylative Suzuki–Miyaura Cross-Coupling of Amides by Carbon–Nitrogen Bond Activation,
Tongliang Zhou, Chonglei Ji, Xin Hong, Michal Szostak,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc03169c