Beryllium is the least-investigated nonradioactive element [1]. This is due to its high toxicity. There is little information, for example, about the chemistry of beryllium in N,N-Dimethylformamide (DMF). This is the case although DMF is widely used and can dissolve beryllium compounds that are insoluble in other solvents.
Matthias Müller and Magnus R. Buchner, University of Marburg, Germany, have performed a comprehensive study on the behavior of beryllium halides in DMF. The team characterized the resulting beryllium species using 1H, 9Be, and 13C NMR spectroscopy, X-ray crystallography, and infrared (IR) spectroscopy. They first treated the anhydrous beryllium halides BeX2 (X = F,Cl,Br,I) with DMF and found that all except BeF2 dissolved readily. From these solutions, crystals of [Be(DMF)4]Cl2, [Be(DMF)4]Br2, and [Be(DMF)4]I2 could be obtained.
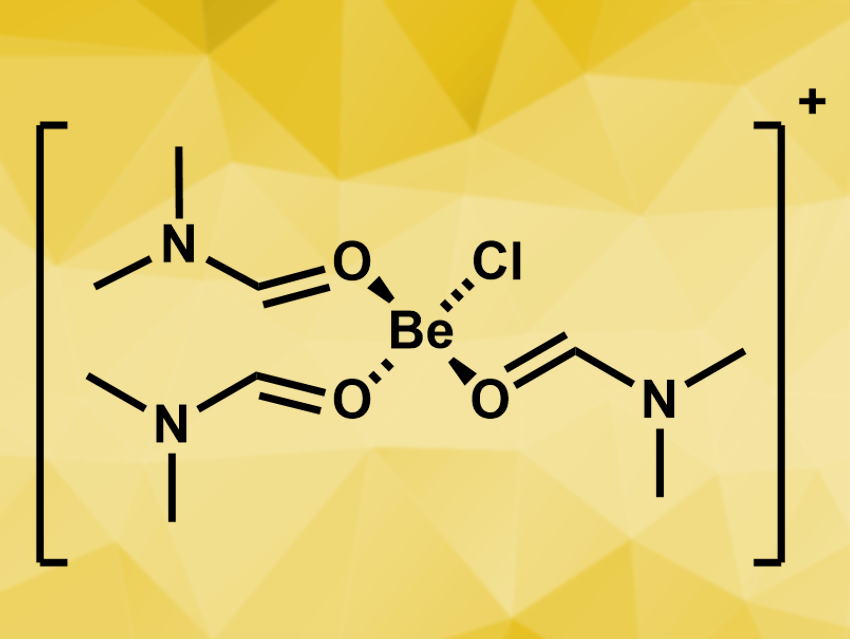
To investigate which other complexes might be formed in solution, the team performed NMR titration experiments, where different amounts of DMF were added to a suspension of BeCl2, BeBr2, or BeI2 in CDCl3. The team found, for example, the dinuclear complexes [BeCl2(DMF)]2 and [BeBr2(DMF)]2, as well as the bis(N,N-dimethylformamide) complexes of beryllium chloride, bromide, and iodide, [BeX2(DMF)2]. The complex cation [BeCl(DMF)3]+ (pictured) was identified as the major component when BeCl2 was dissolved in DMF.
The team also isolated the DMF-bridged dinuclear compounds [Be(DMF)I2]2 and [Be2(DMF)4I2][Be2I6]. According to the researchers, the latter is the first example of a compound with the hexaiodidodiberyllate anion.
- Solution Behavior of Beryllium Halides in Dimethylformamide,
Matthias Müller, Magnus R. Buchner,
Inorg. Chem. 2019.
https://doi.org/10.1021/acs.inorgchem.9b02139
Reference
- [1] Recent Contributions to the Coordination Chemistry of Beryllium,
Magnus R. Buchner,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201901766