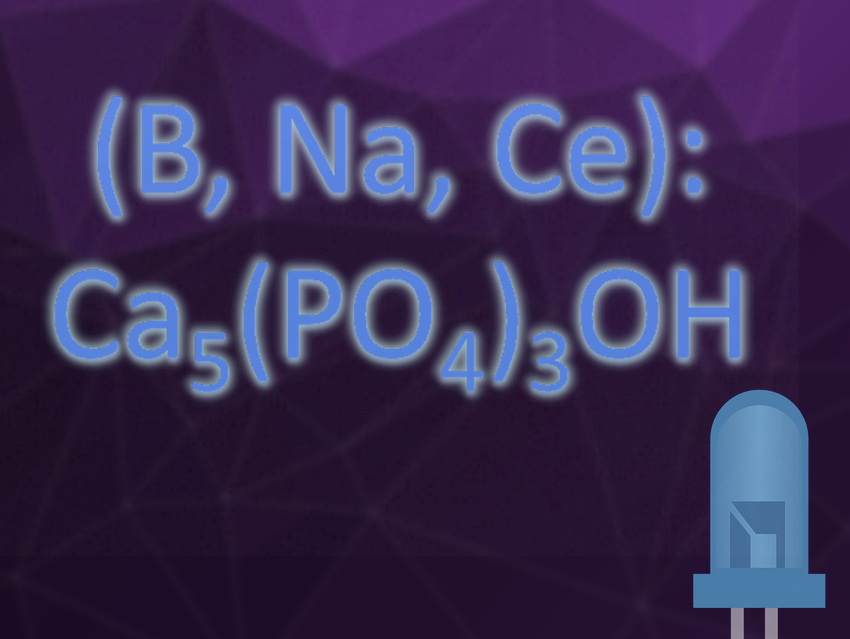
Phosphor-converted light-emitting diodes (pc-LEDs) are a promising technology for energy-efficient lighting. Materials suitable as phosphors for pc-LEDs need an emission spectrum in the visible range, high quantum efficiency, and good thermal stability. Hydroxyapatite (Ca5(PO4)3OH) is usually not an ideal host material for phosphors because the vibrations of the hydroxyl groups reduce or even quench the luminescence.
Jilin Zhang, Shixun Lian, Hunan Normal University, Changsha, China, and colleagues have discovered that a Ce3+-doped hydroxyapatite containing added boron atoms and sodium (for charge compensation) shows intense blue luminescence. The material was synthesized by a high-temperature solid-state reaction. The product was characterized by X-ray powder diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), and elemental analysis. Temperature-dependent photoluminescence and quantum efficiency were measured to test the material’s suitability for use as a pc-LED phosphor.
The FT-IR results indicate the presence of BO32-, while the intensity of the hydroxyl vibration was reduced. X-ray crystallographic data also showed the presence of boron atoms within the host lattice. According to the researchers, the substitution of P5+ by B3+ leads to a decrease of hydroxy groups in the material’s structure to maintain the charge balance. This decrease is responsible for the improved blue emission.
The resulting phosphor provides good quantum efficiency and has a high thermal stability. Therefore, Ce-doped hydroxyapatite containing boron in the host lattice might be a suitable candidate for new LEDs.
- From Nonluminescence to Bright Blue Emission: Boron-Induced Highly Efficient Ce3+-Doped Hydroxyapatite Phosphor,
Xujian Zhang, Jilin Zhang, Wentao Ma, Shuzhen Liao, Xinguo Zhang, Zhengliang Wang, Liping Yu, Shixun Lian,
Inorg. Chem. 2019.
https://doi.org/10.1021/acs.inorgchem.9b02555