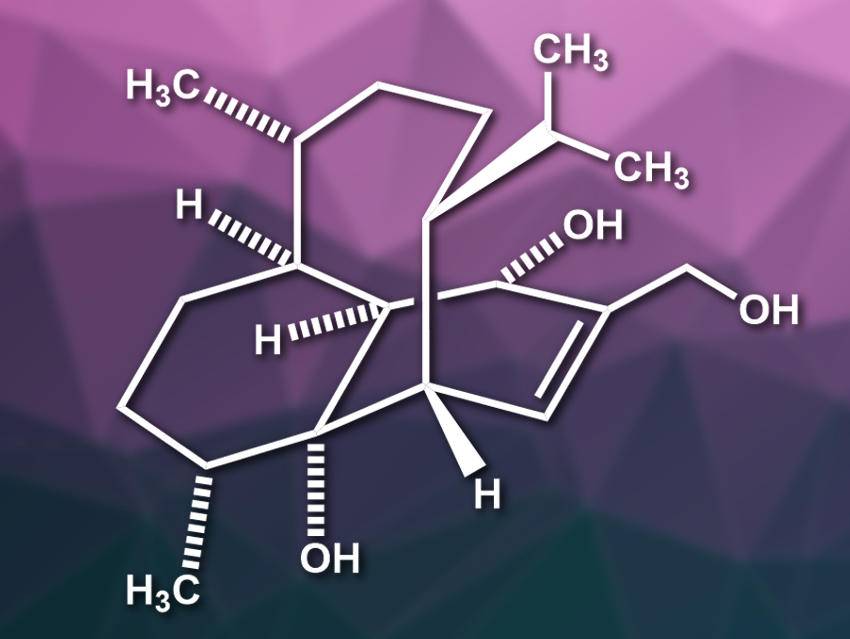
(−)-Vinigrol (pictured) is a structurally complex diterpene and was isolated from a fungus. The compound has an unprecedented rigid 1,5-butanodecahydronaphthalene core with eight contiguous stereocenters. In addition, it contains a bicyclo[5.3.1]-undecane ring system, which is also found in the well-known terpene taxol. Vinigrol has potentially useful biological activities, but is challenging to synthesize.
Chuang-Chuang Li, Southern University of Science and Technology, Shenzhen, China, and colleagues have performed an asymmetric total synthesis of (−)-vinigrol. The team started from commercially available chloro-dihydrocarvone. This starting material was converted to a furane and functionalized using a Grignard reaction and a hydroxymethylation. An oxidative rearrangement, followed by an intramolecular [5+2] cycloaddition, then gives a bicyclo[5.4.1]dodecane skeleton. A ring contraction was the key step in converting this intermediate to the desired (−)-vinigrol.
The synthesis involves a linear sequence of 14 steps from chloro-dihydrocarvone and avoids the need for protecting groups. According to the researchers, it includes the first example of an intramolecular [5+2] cycloaddition to construct eight-membered ring systems in natural product synthesis. The approach should also allow the preparation of further vinigrol analogues.
- Asymmetric Total Synthesis of (−)-Vinigrol,
Long Min, Xiaohong Lin, Chuang-Chuang Li,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b08983

![Cucurbit[7]uril-Based Polymers with Dual Photochromism and Fluorescence](https://www.chemistryviews.org/wp-content/uploads/2025/09/70145-125x94.png)