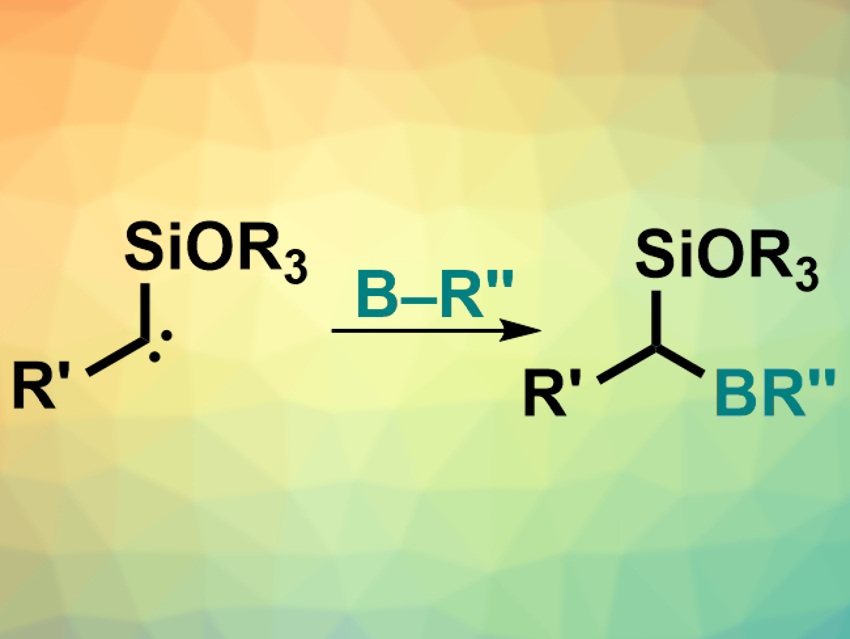
Organoboron compounds are useful intermediates in organic synthesis. Simple, efficient ways of synthesizing organoboron compounds are, thus, interesting. While many other carbon–heteroatom (C–X) bonds can be created via the insertion of a carbene into an X–H bond, such insertions into B–H bonds are challenging.
Frank Glorius, University of Münster, Germany, and colleagues have developed a metal-free insertion of carbenes into B−H bonds, mediated by visible light. The team used electron-rich α-siloxycarbenes (pictured right), which were prepared from acylsilanes under blue light-emitting diodes (LEDs). These carbenes can then directly react with pinacolborane (HBpin) to give the desired α-alkoxyorganoboronate esters.
The reaction is atom-economical and has a broad substrate scope. It tolerates different silyl groups as well as alkyl- and aryl substituents on the α-siloxycarbenes. Almost all products were obtained in quantitative yields, and the reaction is scalable.
- Visible-Light-Induced, Metal-Free Carbene Insertion into B–H Bonds between Acylsilanes and Pinacolborane,
Jian-Heng Ye, Linda Quach, Tiffany Paulisch, Frank Glorius,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b08960