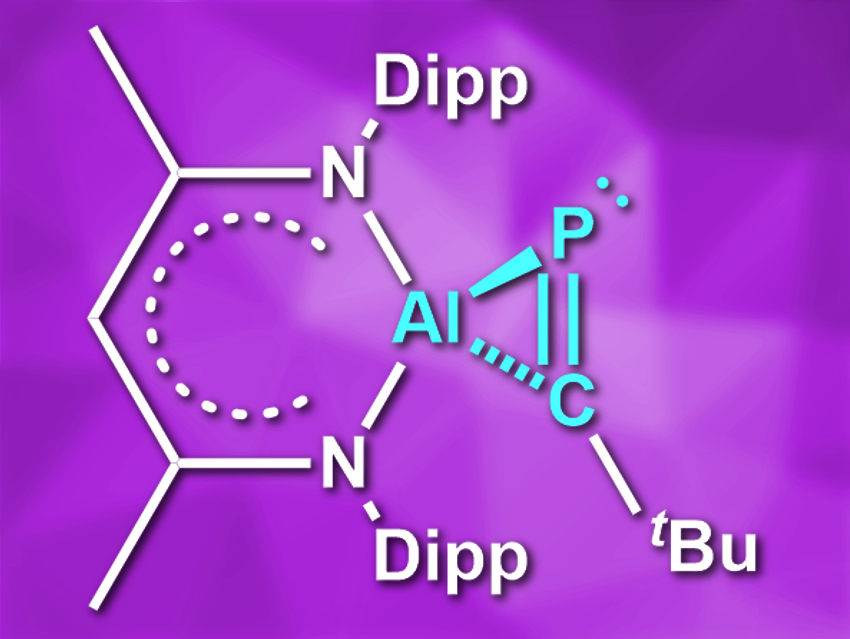
While cyclopropane rings have become very common in organic compounds, three-membered rings with heavier main-group elements are often challenging to synthesize. There are examples of aluminirenes, which contain an AlC2 ring with a double bond, and of phosphirenes, which contain unsaturated PC2 rings. However, no main-group metallaphosphirenes—which contain both phosphorus and a metal atom—had been synthesized so far.
Douglas W. Stephan and colleagues, University of Toronto, ON, Canada, and colleagues have synthesized phosphaaluminirenes (example pictured). The team reacted the Al(I) complex HC[(CMe)(NDipp)]2Al (Dipp = 2,6-iPr2C6H3) with either tBuC≡P or AdC≡P (Ad = adamantyl) in toluene at room temperature and obtained the desired products in yields of 74 % and 70 %, respectively.
The products are the first examples of both heteroalumirenes and metallaphosphirenes. The compounds were characterized using X-ray diffraction as well as 1H, 13C, and 31P NMR spectroscopy, and the bonding situation was investigated using density functional theory (DFT) calculations. According to the researchers, both compounds’ three-membered rings have a moderate three-centered 2π-electron aromaticity. The aluminium atoms in the rings retain Lewis-acidic reactivity.
- Phosphaaluminirenes: Synthons for Main Group Heterocycles,
Liu Leo Liu, Jiliang Zhou, Levy L. Cao, Douglas W. Stephan,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b09330