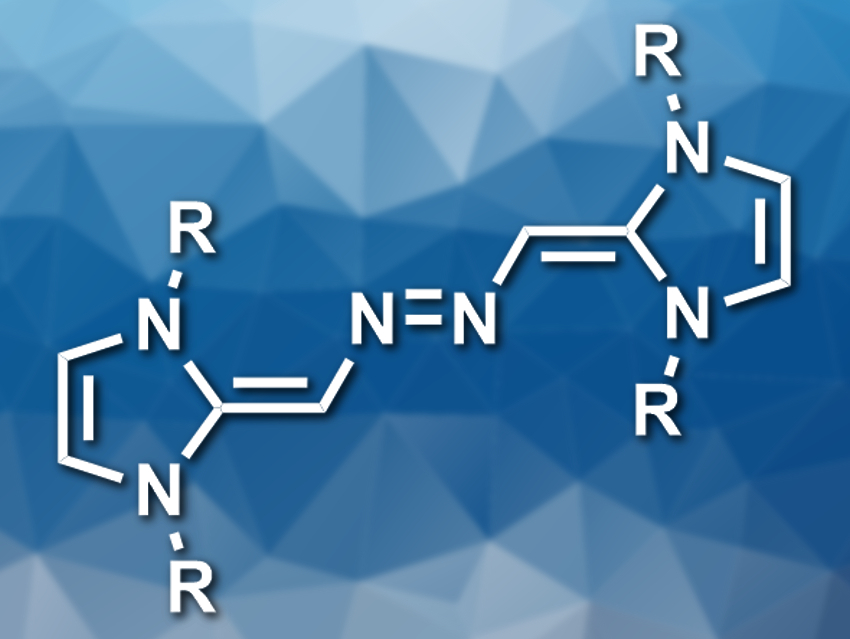
N-heterocyclic carbenes (NHCs) are commonly used as ligands or organocatalysts. N-heterocyclic olefins (NHOs) are the alkylidene derivatives of NHCs. They have a polarized C=C bond that can react with small molecules such as CO2. Nitrous oxide (N2O) is isoelectronic to CO2, so NHOs might also react with N2O.
Kay Severin, École Polytechnique Fédérale de Lausanne (EPFL), Switzerland, and colleagues have investigated this reaction and found that NHOs react with N2O to form electron-rich azo-bridged dimers (pictured). The team used solutions of three differently substituted NHOs (R = 2,6-iPr2C6H3, 2,4,6-Me3C6H2, or 2,6-Me2C6H3) in acetonitrile and exposed them to 1 bar of N2O to give the desired products in yields of about 50 %. The researchers think that the reaction proceeds via zwitterionic NHO–N2O adducts, which tautomerize to give diazohydroxides. These intermediates can undergo a condensation with a second molecule of NHO to give the azo-bridged dimers.
The team found that the products are a new class of organic super-electron-donors: They have a low ionization energy and can easily transfer a single electron. They can be converted into stable radical cations via one-electron oxidations using, e.g., chloroform or benzyl bromide as oxidants. According to the researchers, the compounds have similar reducing properties to tetraazafulvalenes, which are known organic super-reducing agents. Thus, they might have similar applications.
- Synthesis of Organic Super-Electron-Donors by Reaction of Nitrous Oxide with N-Heterocyclic Olefins,
Léonard Y. M. Eymann, Paul Varava, Andrei M. Shved, Basile F. E. Curchod, Yizhu Liu, Ophélie M. Planes, Andrzej Sienkiewicz, Rosario Scopelliti, Farzaneh Fadaei Tirani, Kay Severin,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b10660