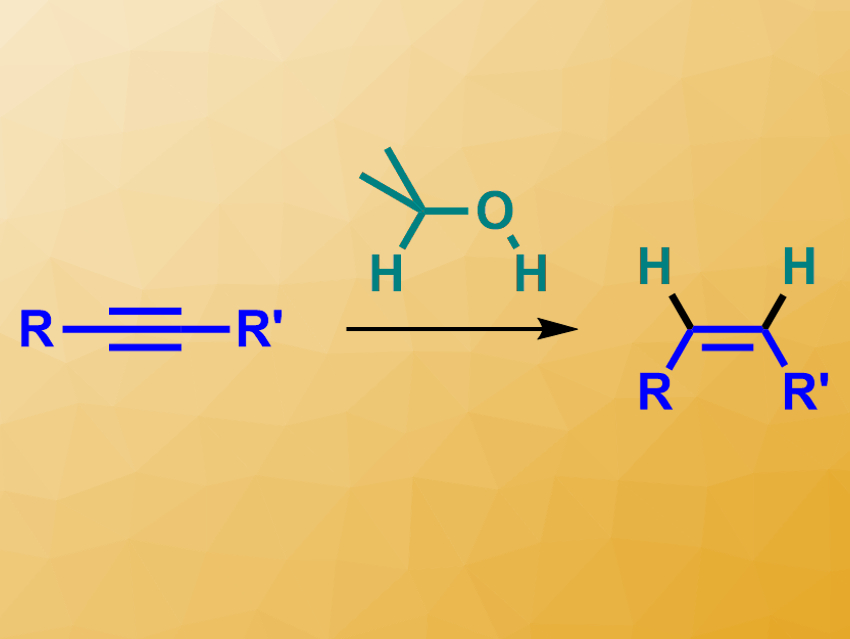
Using hydrogen gas directly in reduction reactions can be problematic due to the need for high-pressure equipment. Catalytic transfer hydrogenations with an H2 equivalent can avoid this issue. Alcohols, for example, can be used to replace H2 in this type of reaction. However, this approach has so far not been used for copper-catalyzed homogeneous transfer hydrogenations. Usually, these processes use hydrosilanes as reducing agents, which generates stoichiometric amounts of silicon-based waste products.
Johannes F. Teichert and colleagues, Technical University of Berlin, Germany, have developed a protocol for the copper-catalyzed transfer semihydrogenation of alkynes (pictured) that uses simple, low-cost alcohols such as isopropanol as hydrogen sources. The team used a complex of the type [NHC–Cu–Cl] as the catalyst (NHC = N-heterocyclic carbene), NaOtBu as a base, and isopropanol as the solvent and H2 equivalent, simultaneously.
The reaction was performed at 140 °C and converted different disubstituted alkynes to the desired alkenes in moderate to excellent yields and with good Z/E-selectivity. The team found no overreduction to the corresponding alkanes. This stereo- and chemoselectivity is attributed to the NHC ligand: When only CuCl is used as the catalyst, selectivities drop significantly and alkanes are formed.
- Using alcohols as simple H2-equivalents for copper-catalysed transfer semihydrogenations of alkynes,
Trinadh Kaicharla, Birte M. Zimmermann, Martin Oestreich, Johannes F. Teichert,
Chem. Commun. 2019.
https://doi.org/10.1039/c9cc06637c